 While the rest of the patent world was focused on Supreme Court opinions (issued and pending) and Congressional action vel non on threats like patent trolls, the consolidated Multi District Litigation between Myriad Genetics and several defendants over BCRA 1 and 2 genetic testing has been proceeding apace in the U.S. District Court for the District of Utah. There have been developments (although none too surprising) that have been to Myriad's benefit, as well as some bad news and the opening of another front in the gene testing wars that threaten to raise the political rhetoric over Myriad's business model to levels reminiscent of the ACLU's tactics.
While the rest of the patent world was focused on Supreme Court opinions (issued and pending) and Congressional action vel non on threats like patent trolls, the consolidated Multi District Litigation between Myriad Genetics and several defendants over BCRA 1 and 2 genetic testing has been proceeding apace in the U.S. District Court for the District of Utah. There have been developments (although none too surprising) that have been to Myriad's benefit, as well as some bad news and the opening of another front in the gene testing wars that threaten to raise the political rhetoric over Myriad's business model to levels reminiscent of the ACLU's tactics.
 The good news for Myriad is that the District Court dismissed two sets of counterclaims: the first, asserted by Ambry Genetics, that the bringing the lawsuit was itself a violation of the antitrust laws. Ambry had argued specifically that Myriad filed its lawsuit "to enforce its invalid patents in bad faith in order to keep competitors from entering the market," citing Myriad's intention to maintain the "status quo" (which, according to Ambry is Myriad's "wrongful monopoly") as set forth in its brief in support of its preliminary injunction motion, in violation of Sections 1 and 2 of the Sherman Antitrust Act. Ambry also asserted as an improper motive for the lawsuit its intention to contribute the results of its BRCA gene testing to public databases. In Ambry's view, Myriad's failure to do the same constitutes an attempt to use "its invalid patents to maintain as secret patients' gene sequences (which do not belong to Myriad) in an attempt to limit competition." And Ambry also asserted that Myriad is using the lawsuit to delay market entry by competitors other than Ambry, and to incorporate aspects of its own technology into Myriad's testing, resulting in an expropriation of "Ambry's superior screening services, which utilize more sensitive, efficient and cost-effective next-generation sequencing technologies." Ambry's counterclaims also alleged that Myriad has "market power" and that its activities have harmed the market for diagnostic genetic testing for BCRA gene mutations in the United States, because Myriad has an "over 90% market share" for BRCA genetic testing and "possesses the power to control prices and exclude competitors." Additionally, Ambry contended that Myriad falsely attested that Ambry's testing has "variant of unknown significance (VUS)" rates of 10-30%, and that Myriad knowing this falsity has had its employees conduct a campaign of informing genetic counselors of this false VUS rate for Ambry's BRCA testing. Myriad opposed these allegations, and moved to dismiss under Fed. R. Civ. Pro. 12(b)(6) because it was immune from antitrust liability under the Noerr-Pennington doctrine first enunciated by the Supreme Court in Eastern R.R. Presidents Conference v. Noerr Motor Freight, Inc., 365 U.S. 127 (1961), and Mine Workers v. Pennington, 381 U.S. 657 (1965). This immunity can only be abrogated by bringing a lawsuit that is objectively baseless, in bad faith or enforcing a patent obtained by fraud, i.e., inequitable conduct.
The good news for Myriad is that the District Court dismissed two sets of counterclaims: the first, asserted by Ambry Genetics, that the bringing the lawsuit was itself a violation of the antitrust laws. Ambry had argued specifically that Myriad filed its lawsuit "to enforce its invalid patents in bad faith in order to keep competitors from entering the market," citing Myriad's intention to maintain the "status quo" (which, according to Ambry is Myriad's "wrongful monopoly") as set forth in its brief in support of its preliminary injunction motion, in violation of Sections 1 and 2 of the Sherman Antitrust Act. Ambry also asserted as an improper motive for the lawsuit its intention to contribute the results of its BRCA gene testing to public databases. In Ambry's view, Myriad's failure to do the same constitutes an attempt to use "its invalid patents to maintain as secret patients' gene sequences (which do not belong to Myriad) in an attempt to limit competition." And Ambry also asserted that Myriad is using the lawsuit to delay market entry by competitors other than Ambry, and to incorporate aspects of its own technology into Myriad's testing, resulting in an expropriation of "Ambry's superior screening services, which utilize more sensitive, efficient and cost-effective next-generation sequencing technologies." Ambry's counterclaims also alleged that Myriad has "market power" and that its activities have harmed the market for diagnostic genetic testing for BCRA gene mutations in the United States, because Myriad has an "over 90% market share" for BRCA genetic testing and "possesses the power to control prices and exclude competitors." Additionally, Ambry contended that Myriad falsely attested that Ambry's testing has "variant of unknown significance (VUS)" rates of 10-30%, and that Myriad knowing this falsity has had its employees conduct a campaign of informing genetic counselors of this false VUS rate for Ambry's BRCA testing. Myriad opposed these allegations, and moved to dismiss under Fed. R. Civ. Pro. 12(b)(6) because it was immune from antitrust liability under the Noerr-Pennington doctrine first enunciated by the Supreme Court in Eastern R.R. Presidents Conference v. Noerr Motor Freight, Inc., 365 U.S. 127 (1961), and Mine Workers v. Pennington, 381 U.S. 657 (1965). This immunity can only be abrogated by bringing a lawsuit that is objectively baseless, in bad faith or enforcing a patent obtained by fraud, i.e., inequitable conduct.
On May 16th, the District Court granted Myriad's motion to dismiss in a terse Order stating that the Order was based on Myriad's argument in its briefing.
 The Court, in an equally terse Order granted Myriad's motion to dismiss Quest Diagnostics' counterclaims for defamation and unfair competition. These counterclaims were based on allegations by Quest that Myriad had, by asserting its patents broadly against any entity entering the BRCA testing market, violated unfair competition laws. Quest's declaratory judgment complaint supported its allegations with factual assertions of specific actions by Myriad representatives.
The Court, in an equally terse Order granted Myriad's motion to dismiss Quest Diagnostics' counterclaims for defamation and unfair competition. These counterclaims were based on allegations by Quest that Myriad had, by asserting its patents broadly against any entity entering the BRCA testing market, violated unfair competition laws. Quest's declaratory judgment complaint supported its allegations with factual assertions of specific actions by Myriad representatives.
The bad news is both general and specific. Generally, Chief Judge Rader's descent from the Federal Circuit bench removes from the Court a strong voice in favor of patent rights and enforcement of valid patents. He consistently applied patent law using traditional concepts until overturned by the Supreme Court (inter alia, in Prometheus v. Mayo) and whatever influence he might have with the other members of the Court is now lost. More bad news stems from the Supreme Court's decisions in Octane Fitness, LLC v. ICON Health & Fitness, Inc. and Highmark Inc. v. Allcare Health Mgmt. Sys., Inc., because (despite the protections of Noerr-Pennington) the District Court might find that bringing these several lawsuits even in good faith engendered liability for all the defendants' litigation expenses.
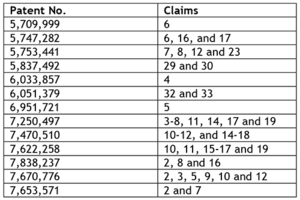
 More significant is the entry into the BRCA gene testing market of Pathway Genomics, who began offering testing for BRCA 1, BCRA 2 and MUTYH genes "on or above June 3, 2014." Myriad filed suit ten days later. In its complaint, which mirrors complaints against several other defendants, Myriad alleges infringement of the following claims of the following patents:
More significant is the entry into the BRCA gene testing market of Pathway Genomics, who began offering testing for BRCA 1, BCRA 2 and MUTYH genes "on or above June 3, 2014." Myriad filed suit ten days later. In its complaint, which mirrors complaints against several other defendants, Myriad alleges infringement of the following claims of the following patents:
Pathway Genomics has announced that its BRCATrue test is one that has taken more than a year to develop and has a sensitivity of 99.99%. Uniquely, and important for the politics of the situation is Pathway Genomic's decision to donate up to $10 million in "free" genetic testing, in partnership with eight advocacy groups including the Susan G. Koman for the Cure, Bright Pink, Living Beyond Breast Cancer, National Ovarian Cancer Coalition, Young Survival Coalition, Foundation for Women's Cancer, Facing Our Risk of Cancer, and Sharsheret. But this generosity is grounded on sound economics: each "free" test will only be given one-for-one with paid testing. Still, in view of the cost for each "paid-for" test of $1,799, the $10 million represents about 5,600 "free" tests. And while Pathway Genomic's price is significantly less than Myriad's on its face (Myriad's tests cost $3,000-4,000 apiece), Myriad's business model is to charge relatives of a patient much less than the test for the initial patient. Thus, it may be possible that for a given family Myriad's test costs could well be less than Pathway's.
Despite these potentialites, Pathway Genomics has seized the rhetorical high ground (or low, depending on your understanding of the issues). A press release issued by Pathway Genomics after Myriad filed suit proclaims "Pathway Genomics Responds to Myriad Genetics Lawsuit, Defends the Rights of All Women to Receive BRCA Testing Regardless of Socioeconomic Status" and is illustrated with a graphic that would have been at home in a World War II propaganda banner flying over the Red Army:

These graphics are accompanied by strong words from Jim Plante, Pathway Genomics' CEO, who the press release quotes as saying:
We are a company trying to do good in the world. Forty-eight hours after we raised hundreds of thousands of dollars for Susan G. Komen to fight breast cancer and announced our donation of $10 million of free genetic testing for women in need, Myriad Genetics slaps us with this unwarranted lawsuit.
Given their pattern of filing lawsuits against other companies broadening access to this life-saving technology in clear disregard of the Supreme Court's decision last year, this lawsuit is not unexpected. We do not infringe on any valid patent claims and are prepared to vigorously defend ourselves.
And almost obligatorily:
We are on a mission to make genetic testing more accessible and affordable, especially testing related to cancer screening, one of the leading causes of death in the world. We believe that no corporation should have a monopoly over an individual's genes. Every person should have access to vital information about their own body.
In addition, Pathway Genomics has broached the issue of Myriad's sequestration of its genetic diagnostic mutation database, which Myriad has kept proprietary since about 2004. Pathway Genomics also touts in its press release that it is part of the "Free the Data" movement, which opposes keeping genetic information as trade secrets (see "Consortium Launches Public Database of BRCA Data"). Their asserted hope is that "broadening access and sharing genetic information, we can collectively harness the power of the genome to improve our understanding of disease and improve healthcare for everyone" and vows to make its BRCA gene data available on the public database ClinVar. While Myriad today has a large head start in its proprietary database, rapid adoption of Pathway Genomics' testing may provide a viable commercial alternative in much less time than it has taken Myriad to develop its own database (and may influence how long Myriad keeps its database as a trade secret). These efforts may provide a market alternative to proposals for Congress to mandate disclosure, which would of course constitute a taking under the Fifth Amendment of the Constitution and require compensation sufficient to vitiate the public health cost advantage its proponents advocate.