 Ever since the Supreme Court handed down its decision in KSR Int'l Co. v. Teleflex Inc., 550 U.S. 398 (2007), both the U.S. Patent and Trademark Office and the courts have found it easier to render a decision that a claimed invention was obvious. While how the USPTO and the courts have implemented the KSR obviousness standards have been the subject of some debate, occasionally there is a case that illustrates that the nuances of the Court's obviousness rubrics can be applied in such a way that comports with the "common sense" elevated to an analytical tool by the Court in KSR.
Ever since the Supreme Court handed down its decision in KSR Int'l Co. v. Teleflex Inc., 550 U.S. 398 (2007), both the U.S. Patent and Trademark Office and the courts have found it easier to render a decision that a claimed invention was obvious. While how the USPTO and the courts have implemented the KSR obviousness standards have been the subject of some debate, occasionally there is a case that illustrates that the nuances of the Court's obviousness rubrics can be applied in such a way that comports with the "common sense" elevated to an analytical tool by the Court in KSR.
Such a case arose in the application of Doran Adler et al. The case involved an appeal of a PTO determination that the claims of USSN 10/097,096 to applicants Doran Adler et al. were obvious. The invention relates generally to methods and systems for detecting "pathologies of the gastrointestinal tract"; claim 57 is representative:
57. A method for displaying in-vivo information, the method comprising:
receiving at a data processor data generated by a swallowable in-vivo device transversing a GI tract, the data comprising a set of in-vivo images of the GI tract;
the data processor comparing values of the received images to a reference value of blood and to a reference value of healthy tissue;
the data processor causing to be displayed the imaged as a color video; and
the data processor further, based on the comparison, causing to be displayed an indication of a position in the GI tract of a change in the level of red color content, the change correlating to the presence of blood.
Also at issue (but not separately argued) was a system claim (claim 63), substantially comprising a system for practicing the method of claim 57. The cited prior art was the Meron (WO 00/22975) reference, which discloses a swallowable camera comprising a "means for sensing the presence of blood" combined with the Hirata reference (a scientific journal article) that discloses an endoscopic comparison of normal and healthy esophageal tissues based on colorometric analysis of esophageal varicies detected by comparing "color toner and red color sign."
The PTAB affirmed the Examiner's obviousness rejection, in a non-precedential decision by APJ Grimes, joined by APJ's Scheiner and Mills, finding that despite the absence of the use of the Meron system to detect blood, the combination of Meron's teachings (that a video camera could be miniaturized and used to detect GI anomalies "that may be associated with bleeding" in the GI tract) with Hirata's disclosure that image comparison could be used to detect changes in the red color spectrum of the GI tract led to a conclusion of obviousness.
The Federal Circuit affirmed, in an opinion by Judge Wallach joined by Judges Prost and Reyna. The panel cited specific findings of fact that the opinion held were supported by substantial evidence:
12. Hirata discloses that color tone was analyzed by comparing the color tone of a defined varices region with the color tone of a defined normal esophageal region.
13. Hirata discloses that the area of red color sign was also determined for a defined varices region.
14. Hirata discloses that, using image processing, both color tone results and area of red color sign results could be used to select patients with varices that have a higher risk of rupture.
(It was uncontested that the Meron reference teaches a swallowable camera.) The Court found that the Board did not err in its obviousness determination, and that the Board did not base its obviousness determination on grounds different from those relied upon by the Examiner (which would have entitled applicants to a rehearing on the merits). As set forth in the panel's opinion:
The primary issue on appeal is whether the Board properly found that it would have been obvious in light of the prior art to compare reference values for healthy tissue and blood to determine whether images of the gastrointestinal tract showed "a change in the level of red color content" where that "change correlat[es] to the presence of blood," as articulated in the claims at issue.
The appellants argued that their claims embody two comparisons (between patient images and an image of healthy tissue or between patient images and blood). The Court affirmed the PTO's conclusion that a "two-prong" assessment as advocated by appellants was merely a "predictable variation" on the combination of Hirata and Meron and thus unpatentable for being obvious, citing KSR Int'l Co. v. Teleflex Inc., 550 U.S. 398, 417 (2007) ("If a person of ordinary skill can implement a predictable variation, § 103 likely bars its patentability."). The opinion rejected appellants' attempts to distinguish the Hirata reference for failing to teach detection of blood, stating (in a footnote) that "substantial evidence supports the Board's finding that Hirata teaches identification of a pathology through red color image analysis of two reference values" wherein "one of ordinary skill in the art would understand that detecting areas with different red color values corresponds to blood."
The purported "new grounds of rejection" argument was predicated on appellants' contention that the Board's analysis "changed the thrust of the Examiner's rejection (Hirata's classification of red color signs); the opinion reproduces appellants comparison between the Examiner's grounds of rejection and the rationale set forth in the PTAB's decision:
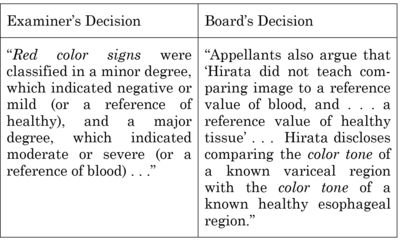 The court found that appellants did not identify "specific facts found by the Board but not by the examiner, nor [did they] illustrate[] how any such facts formed the basis of the Board's rejection." Accordingly:
The court found that appellants did not identify "specific facts found by the Board but not by the examiner, nor [did they] illustrate[] how any such facts formed the basis of the Board's rejection." Accordingly:
While the Board's explanation may go into more detail than the examiner's, that does not amount to a new ground of rejection. See In re Jung, 637 F.3d 1356, 1365 (Fed. Cir. 2011).
In re Adler (Fed. Cir. 2013)
Panel: Circuit Judges Prost, Reyna, and Wallach
Opinion by Circuit Judge Wallach