 Representatives from the U.S. Patent and Trademark Office, led by Janet Gongola, Senior Advisor to the Deputy Director of the U.S. Patent and Trademark Office, spent the better part of last month traversing the U.S. presenting a Roundtable on the activities and preferred modes of practice before the Patent Trial and Appeal Board, the successor under the Leahy-Smith America Invents Act for the Board of Patent Appeals and Interferences. The presentation included a talk by Chief Judge James Smith on the state of practice before the Board (including a wealth of statistics and goals) and panel discussion between members of the local bar and members of the Board (see "PTAB AIA Trial Roundtables" webpage).
Representatives from the U.S. Patent and Trademark Office, led by Janet Gongola, Senior Advisor to the Deputy Director of the U.S. Patent and Trademark Office, spent the better part of last month traversing the U.S. presenting a Roundtable on the activities and preferred modes of practice before the Patent Trial and Appeal Board, the successor under the Leahy-Smith America Invents Act for the Board of Patent Appeals and Interferences. The presentation included a talk by Chief Judge James Smith on the state of practice before the Board (including a wealth of statistics and goals) and panel discussion between members of the local bar and members of the Board (see "PTAB AIA Trial Roundtables" webpage).
While concerned generally with the Board's activities, a substantial portion of the program related to inter partes review (IPR), the successor under the AIA of inter partes reexamination. 35 U.S.C. §§ 311-319. The Board members discussed with members of the local bar best practices and the bar's experiences with IPR, and included one or more role-playing sessions. One of these, on how a patentee party can best present a motion to amend the claims incited insightful comment from the audience wherever the Roundtables were held. In Chicago, the issue was presented to the Board members by Edward Manzo, a partner at Husch Blackwell (and editor of Claim Construction and the Federal Circuit, a yearly tome on how the Court has decided claim construction issues). Mr. Manzo presented his question thusly (paraphrased here):
Because the PTAB has determined that it will apply the broadest reasonable interpretation (BRI) to the claims under inter partes review, and further has decided that it will not make an independent determination of patentability (unlike in the former inter partes reexamination); and further because the patentee does not have the right to amend the claims but must get leave to amend from the Board by motion, doesn't this refusal to freely permit amendment abolish the justification usually proffered for applying BRI, specifically, that the Office uses this standard because the applicant/patentee has the ability to amend to overcome rejection based on BRI?
(The question is particularly germane because at the time Mr. Manzo asked it the Board had not granted a single motion for leave to amend.) The answer given by the Board had much to do with the statutory time limitation for bringing IPR to a conclusion (one year from granting the petition for IPR, with an ability to extend that time "for good cause," a provision the Board clearly does not want to invoke routinely); and the burden on PTAB resources if the Board decided patentability issues; and the ability of the patentee to respond to the petition with counter-arguments; and the ability of the parties to settle (while conceding that once the Board established that the public interest was implicated it might decide, and had the statutory capacity to decide, the substantive patentability questions raised by the IPR even if the parties decided to settle).
One intriguing aspect of the Board's response to Mr. Manzo was a comment by the Chief Judge, to the effect that we should all "stay tuned." The basis for this comment became evident on Tuesday, May 20th when the Board issued its first grant of a motion for leave to amend, in International Flavors and Fragrances v. U.S. (Final Written Decision). The patent at issue, U.S. Patent No. 7,579,016, is entitled "Methods for repelling arthropods using isolongifolenone analogs." The PTAB granted the petition for inter partes review on June 27, 2013 as to claims 1, 4, 5, 7, 8, and 14-26; independent claim 1 reads as follows:
1. A method for repelling arthropods, said method comprising treating an object or area with an arthropod repelling effective amount of at least one isolongifolenone analog and optionally a carrier or carrier material; wherein said at least one isolongifolenone analog has the following formula:
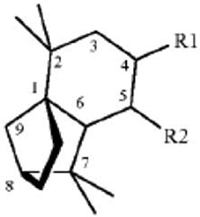
wherein R1 is hydrogen, an oxygen, a C1-10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol with a C1-10 saturated or unsaturated, straight or branched acid and R2 is hydrogen, an oxygen, a C1-10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol with a C1-10 saturated or unsaturated, straight or branched acid; optionally there is a double bond between carbons 5 and 6 and R2.
The Motion to Amend cancelled all claims in the patent (claims 1-26) and to substitute new claims 27-45. The Petitioner, International Flavors and Fragrances, did not oppose, and the Board granted the motion in part and issued its final decision in the opinion handed down on May 20th.
The Board granted the Petition based on the Behan reference, PCT International Publication Number WO 00/19822, published April 13, 2000, which despite failing to demonstrate operability disclosed the use of isolongifolanone (a compound related to the claimed isolongifolenone) to repel arthropods and thus anticipated claims 1, 4, 5, 7, 8, 14, 19-21, and 23. The Board also granted the Petition with regard to claims 1, 4, 5, 7, 8, 14-21, and 23 as being obvious over the combination of the Behan reference and the Grieco reference (A Novel High-Throughput Screening System to Evaluate the Behavioral Response of Adult Mosquitoes to Chemicals, 21 J. AM. MOSQUITO CONTROL ASS'N 404-411 (2005)), which provided disclosure relating to the expressly recited amounts and concentrations of isolongifolenone, and for obviousness as to claims 1, 4, 5, 7, 8, 14, and 19-26 over the combination of the Behan reference and the Carroll reference (Repellency of Deet and SS220 applied to skin involves olfactory sensing by two species of ticks, 19 MEDICAL AND VETERINARY ENTOMOLOGY 101-106 (2006)), which supplies disclosure regarding the expectation that compounds effective against mosquitoes would be effective against other arthropods, such as ticks.
The substitute claims proposed by patentee limited independent claim 27 (corresponding to original claim 1) to five of the compounds recited in the Markush group found in original claim 8 of the '016 patent, and proposed claim 45 to be limited to mites and ticks but otherwise identical to proposed claim 27. These amendments and differences are set forth below:
27. A method for repelling arthropods, said method comprising treating an object or area with an arthropod repelling effective amount of at least one isolongifolenone analog and optionally a carrier or carrier material; wherein said at least one isolongifolenone analog has the following formula:
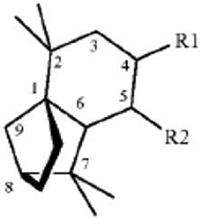
wherein R1 is hydrogen, an oxygen, a C1-10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol with a C1-10 saturated or unsaturated, straight or branched acid and R2 is hydrogen, an oxygen, a C1-10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol with a C1-10 saturated or unsaturated, straight or branched acid; optionally there is a double bond between carbons 5 and 6 and R2; wherein said at least one isolongifolenone analog is selected from the group consisting of
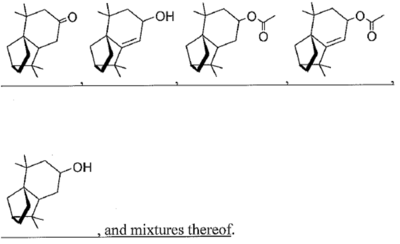
45. A method for repelling arthropods, said method comprising treating an object or area with an arthropod repelling effective amount of at least one isolongifolenone analog and optionally a carrier or carrier material; wherein said at least one isolongifolenone analog has the following formula:
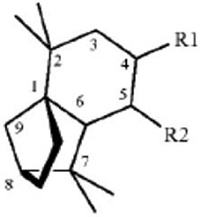
wherein R1 is hydrogen, an oxygen, a C1-10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol with a C1-10 saturated or unsaturated, straight or branched acid and R2 is hydrogen, an oxygen, a C1-10 alcohol, aldehyde, alkyl, ether, or esters of said alcohol with a C1-10 saturated or unsaturated, straight or branched acid; optionally there is a double bond between carbons 5 and 6 and R2; wherein said arthropods are ticks or mites.
The Board set forth the standards it applies to Motions for Leave to Amend during inter partes review:
As the moving party, Patent Owner bears the burden of proof to establish that it is entitled to the relief requested. 37 C.F.R. § 42.20(c). The proposed amendment is not entered automatically, but only upon Patent Owner having demonstrated, by a preponderance of the evidence, the patentability of the proposed claims. See, e.g., 37 C.F.R. § 42.1(d) (noting that the "default evidentiary standard [in proceedings before the Board] is a preponderance of the evidence.").
In addition, the Decision notes that any amendment cannot enlarge or broaden the scope of the patented claims, citing 35 U.S.C. § 316(d)(3); 37 C.F.R. § 42.121(a)(2)(ii). The proposed amendments do not run afoul of this proscription, because they limit the scope of patented claim 1.
The Board also noted that proposed amended or substitute claims must have support under 35 U.S.C. § 112 throughout its scope. 37 C.F.R. § 42.121(b)(1). The proposed substitute claims satisfy this requirement, the Board concluded, based on citation to express disclosure in the specification.
Finally, the Board addressed the patentability of the proposed substitute claims over the cited art. However:
Distinguishing the proposed claims only from the prior art references applied to the original patent claims is insufficient to demonstrate patentability over prior art. As the moving party, a patent owner bears the burden to show entitlement to the relief requested. 37 C.F.R. § 42.20(c).
Support for this proposition is drawn from the Board's earlier decision in Free Sys., Inc. v. Bergstrom, Inc., IPR2012-00027, slip op. at 33 (PTAB January 7, 2014 (Paper 66)), which specified that "the patent owner bears the burden of proof to demonstrate patentability of the proposed claims over the prior art in general, and thus entitlement to the proposed claims." In practice, this imposes the burden that "the patent owner should discuss, as well as present evidence, if appropriate, as to the level of ordinary skill in the art, and what was known regarding the features being relied upon to demonstrate patentability of the proposed claims."
Here, the Board set forth in its decision a synopsis of the evidence presented by the patent owner regarding the unpredictable effects in function that even small changes in chemical structure can have, and concluded that '"[b]ecause the prior art does not provide a reason to modify isolongifolanone to arrive at the modified isolongifolanone compounds of proposed claim 27, nor does it provide a reasonable expectation that such modifications would result in a compound having the desired insect repellent activity, we conclude that the preponderance of the evidence supports the patentability of claim 27. As to dependent claims 28-44, because those claims incorporate all of the limitations of claim 27, they would be patentable for the same reasons."
The Board did not reach the same conclusion of patentability of proposed claim 45 over the prior art. For this claim, the recited structure encompasses the structure disclosed in the Behan reference, which teaches the use of isolongifolanone against mosquitoes. Illustrating the burden the Board imposes on patent owners, the decision states that "Patent Owner provides no evidence that ticks and mites would not be present on the same objects or areas where mosquitoes and cockroaches are found, which are the insects addressed by Behan. Thus, by applying the isolongifolanone taught by Behan to an object or airspace for the purpose of repelling mosquitoes or cockroaches, one would also inherently repel ticks and mites." From this, the Board applies the black-letter principle of the law that "'[i]t is a general rule that merely discovering and claiming a new benefit of an old process cannot render the process again patentable," citing In re Woodruff, 919 F.2d 1575, 1578 (Fed. Cir. 1990); Perricone v. Medicis Pharm. Corp., 432 F.3d 1368, 1377-78 (Fed. Cir. 2005) and Bristol-Myers Squibb Co. v. Ben Venue Laboratories, Inc., 246 F.3d 1368, 1376 (Fed. Cir. 2001) in support. Accordingly, the Board "conclude[d] that the preponderance of the evidence does not support the patentability of proposed claim 45."
Because grant of the Motion, and the reasons therefore are dispositive of all issued before the Board, the decision was made final and the Board cited 37 C.F.R. § 90.2 for the parties' responsibilities for notice and service of judicial review.
A few rubrics can be drawn from this decision. The substitute claims were not challenged by the Petitioner (no doubt because the Petitioner was not at risk of being sued for infringement of the proposed substitute claims); this situation is not likely to be the norm. In addition, the patentee was the U.S. government, which may take a different view of how best to protect its intellectual property than commercial concerns. Petitioner supported its petition using a prior art reference that anticipated the claims, which may or may not be or become routine but certainly provides strong support for satisfying Petitioner's burden of making a showing that at least one claim is unpatentable. Finally it cannot escape the patent community's notice that the Office is exquisitely sensitive to criticism (for example, from the Supreme Court as evidenced by the recently issued subject matter eligibility Guidelines); the Board has been roundly criticized, by the bar and influential members of the judiciary including recent Chief Judge Rader (who called the PTAB a "death squad" for patent claims) as being inimical to patents and patentees based on its high rate of granting IPR petitions and the percentage of those claims that survived (indeed, the Office recently trumpeted the statistic that 41% of patents in IPR survived with the patentability of at least "some" claims being affirmed). Grant of patent owner's Motion for Leave to Amend proves that the Board can permit a patentee under appropriate circumstances to propose claim-saving amendments; the question remaining is whether this becomes routine or remains the black swan of PTAB proceedings.