Robotic technology has been used in medicine for over 30 years. In 1985, a robot called the Puma 560 (shown at right) was used to perform a needle biopsy of human brain tissue. By the turn of the last century, it was widely recognized that robots could provide increased precision, stability, and dexterity in the operating room. The technology has since led to many advances in minimally invasive surgery, which allows procedures to be performed through one or more small incisions or ports. With smaller incisions come benefits such as decreased pain, blood loss, and recovery time.
Robotic technology has been used in medicine for over 30 years. In 1985, a robot called the Puma 560 (shown at right) was used to perform a needle biopsy of human brain tissue. By the turn of the last century, it was widely recognized that robots could provide increased precision, stability, and dexterity in the operating room. The technology has since led to many advances in minimally invasive surgery, which allows procedures to be performed through one or more small incisions or ports. With smaller incisions come benefits such as decreased pain, blood loss, and recovery time.
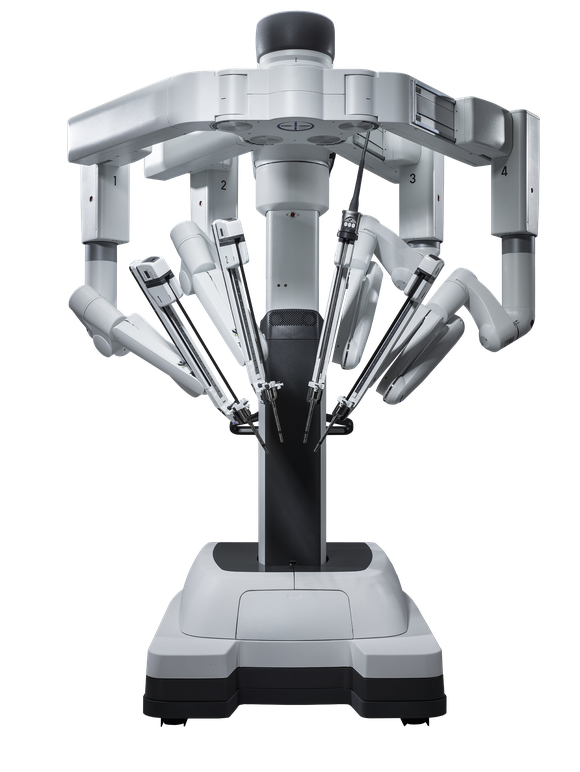 According to Dr. Eric J. Moore, a head and neck surgeon at the Mayo Clinic, the most widely used robotic surgical device is the da Vinci system (shown at left) manufactured by Intuitive Surgical (NASDAQ: ISRG) based in Sunnyvale, CA. The da Vinci was the first robotic system to be FDA approved for general laparoscopic procedures. It was introduced in 1999 and is currently used to treat a variety of conditions including prostate cancer, inflammatory bowel disease, and mitral valve prolapse. This January the FDA approved an integrated motion table, developed in collaboration with Trumpf Medical, for use with the da Vinci system. It allows surgeons to reposition the patient during a procedure without having to disconnect the surgical robotic arms. Earlier this month, the FDA approved Intuitive Surgical’s da Vinci Xi single-site instruments and accessories, which permit surgeons to perform single and multi-port surgeries using one robotic system. Intuitive Surgical holds over 100 patents on surgical robotic systems, software, parts, and accessories.
According to Dr. Eric J. Moore, a head and neck surgeon at the Mayo Clinic, the most widely used robotic surgical device is the da Vinci system (shown at left) manufactured by Intuitive Surgical (NASDAQ: ISRG) based in Sunnyvale, CA. The da Vinci was the first robotic system to be FDA approved for general laparoscopic procedures. It was introduced in 1999 and is currently used to treat a variety of conditions including prostate cancer, inflammatory bowel disease, and mitral valve prolapse. This January the FDA approved an integrated motion table, developed in collaboration with Trumpf Medical, for use with the da Vinci system. It allows surgeons to reposition the patient during a procedure without having to disconnect the surgical robotic arms. Earlier this month, the FDA approved Intuitive Surgical’s da Vinci Xi single-site instruments and accessories, which permit surgeons to perform single and multi-port surgeries using one robotic system. Intuitive Surgical holds over 100 patents on surgical robotic systems, software, parts, and accessories.
The global market for surgical robotic systems is expected to grow significantly in the next 5 years. It was estimated to be worth $3.3 billion in 2014 and is estimated to reach $6.4 billion by 2020. In recent years, several competitors have emerged to challenge Intuitive Surgical, which according to Reuters, holds a dominant position in the surgical robotics market. TransEnterix (NYSE: TRXC) is a medical device company in the Research Triangle area of North Carolina. TransEnterix is currently seeking 510(k) clearance from the FDA for its SurgiBot single-port robotic surgery platform. The company also markets a multi-port system called the ALF-X.
Last year Google’s Verily and Ethicon, a medical device company owned by Johnson & Johnson, partnered to form Verb Surgical of Mountain View, California. Though no specific products have been announced by Verb Surgical, the company’s website promises to create the future of surgery, which will involve robots, advanced imaging technology, and machine learning. Verb has reportedly hired over 100 people including a former CEO of the Volcano Corporation, which specializes in intravascular imaging.
![AR-141209891[1]](https://jdsupra-html-images.s3-us-west-1.amazonaws.com/c1e876ad-4d93-41d7-80a8-3b9a9fddaa35-AR-1412098911.jpg) Since the origin of medical robotics over 30 years ago, robots have migrated out of the operating room and into the recovery room. Assistive robotic systems help people with disabilities accomplish activities of daily living such as eating and walking. They can also be used to speed recover from stroke or other causes of paralysis.
Since the origin of medical robotics over 30 years ago, robots have migrated out of the operating room and into the recovery room. Assistive robotic systems help people with disabilities accomplish activities of daily living such as eating and walking. They can also be used to speed recover from stroke or other causes of paralysis.
Recent advances have made assistive robotics lighter and more versatile. Last month, the FDA approved a lightweight robotic exoskeleton to give increased mobility to people with paraplegia (lower body paralysis). The device, called the Indego (shown at left), was developed at Vanderbilt University’s Center for Intelligent Mechatronics. It will be produced by the Parker Hannifin Corporation (NYSE: PH), which specializes in motion and control technologies. The Indego is reported to weigh only 26 pounds and is designed for easy access to and from a wheelchair. As part of the FDA approval process, the device underwent clinical testing in which it was used on a variety of different surfaces.
Professor Michael Goldfarb whose team developed the Indego describes it as a Segway with legs. If the wearer leans forward, then the Indego moves forward. If she leans back and holds that position, then the Indego and the wearer sit down. The amount of assistance provided by the device can vary to accommodate users with different levels of muscle control. Vanderbilt has filed a US patent application on a movement assistance device.
 The Indego is the second device to be approved by the FDA for people with paraplegia. In June of 2014, the agency approved the ReWalk for similar applications. The Rewalk is manufactured by Rewalk Robotics (NASDAQ: RWLK) an Israeli corporation with its US headquarters in Marlborough, MA. The company made an initial public offering in September of 2014. It is the assignee of record of a US patent on a locomotion assisting device and method (shown at right), a motorized exoskeleton unit, and a locomotion assisting apparatus with integrated tilt sensor.
The Indego is the second device to be approved by the FDA for people with paraplegia. In June of 2014, the agency approved the ReWalk for similar applications. The Rewalk is manufactured by Rewalk Robotics (NASDAQ: RWLK) an Israeli corporation with its US headquarters in Marlborough, MA. The company made an initial public offering in September of 2014. It is the assignee of record of a US patent on a locomotion assisting device and method (shown at right), a motorized exoskeleton unit, and a locomotion assisting apparatus with integrated tilt sensor.
Medical robots have traditional been made using rigid materials. However, engineers have been experimenting with soft materials that interface more comfortably with the human body. This work has created a new field of engineering called soft robotics that uses softer materials like textiles, polymers, and elastomers to create robotic devices. Many designs in this field have been inspired by structures found in nature such as the body of an eel or the tentacles of an octopus. Researcher’s at MIT have used the technology to develop a soft robotic gripper (shown below) that is capable of grasping and identifying more objects than traditional robotic graspers.

Harvard’s Biodesign Lab is developing gloves that incorporate soft robotics to help stroke survivors recover from their injuries. The lab is also developing soft exosuits, which are wearable robots that conform to the human body and are less obtrusive than traditional exosuits. These devices can assist patients with neurologic or muscular conditions, or they can augment the natural abilities of healthy individuals. Much of this research is funded by the Defense Advanced Research Projects Agency (DARPA), a research arm of the U.S. Department of Defense. In collaboration with Harvard’s Wyss Institute, DARPA is developing soft, lightweight exoskeletons to help soldiers carry heavy loads. Harvard currently has patents pending on soft robotic actuators, a soft exosuit for assistance with human motion, a magnetic assembly of soft robots with hard components, and systems and methods for modular soft robotics.
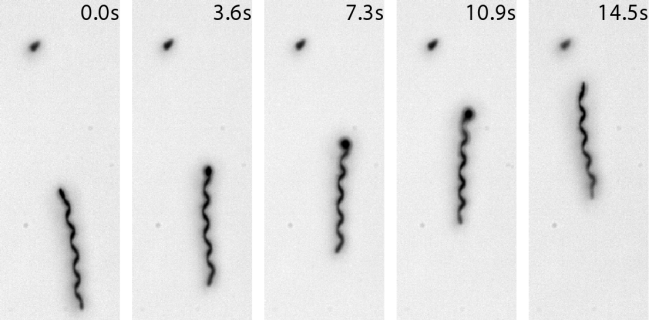
Robots in surgery, medicine, and rehabilitation have come a long way since their origin over a quarter century ago. In the near future, we are likely to see smaller surgical incisions and less obtrusive wearable robots that will be integrated into our clothing. In addition to helping treat disease or disability, robots will increasingly augment the abilities of healthy individuals to increase performance. In the more distant future, micro or nan0-scale robots (shown below swimming) may be employed to perform biopsies or surgery from within our bodies.