 Interferences are not yet dead, although their echo is expected to attenuate over the next decade or so as a result of the provisions of the Leahy-Smith America Invents Act that converted the U.S. from a first-to-invent country to a first-inventor-to-file country. In Tobinick v. Olmarker, the Federal Circuit addressed one of the more arcane points of interference practice, relating to whether a claim in interference is supported by an adequate written description.
Interferences are not yet dead, although their echo is expected to attenuate over the next decade or so as a result of the provisions of the Leahy-Smith America Invents Act that converted the U.S. from a first-to-invent country to a first-inventor-to-file country. In Tobinick v. Olmarker, the Federal Circuit addressed one of the more arcane points of interference practice, relating to whether a claim in interference is supported by an adequate written description.
The circumstance arises when an applicant "provokes" an interference by copying a claim of third party application or patent. In order to prevent a party from harassing a patentee with a frivolous interference, the rules provide that whether those claims are supported by an adequate written description is a threshold issue that can strip the provoking party from standing to be involved in an interference. 37 C.F.R. § 41.201(2)(ii). As the Federal Circuit opinion explains:
In interference proceedings, a disputed claim is construed in the context of its originating disclosure rather than the interfering application. Robertson v. Timmermans, 603 F.3d 1309, 1312 (Fed. Cir. 2010) ("When a party challenges written description support for an interference count or the copied claim in an interference, the originating disclosure provides the meaning of the pertinent claim language.") [internal citations omitted]. Here, the claim limitation "wherein the antibody is administered locally" is construed in light of the '995 and '990 patent specifications, not the '205 application.
The claims at issue in the interference (No. 105,866) are generally directed to methods for alleviating symptoms of a nerve disorder arising from injured spinal disks by administering inhibitors of tumor necrosis factor-a (TNF-a). This invention was based on the finding that injured spinal disks leaked nucleus pulposus into the epidural space of the spine, and that nucleus pulposus contains TNF-a, which creates inflammation in the nervous tissues of the spine, including the peripheral nerve roots sprouting from the spine and traversing the epidural spaces. Senior Party Olmarker had claims from five patents involved in the interference as follows:
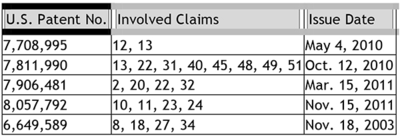
The interference counts were identical to claims from Junior Party Tobinick's patent application, U.S. Serial No. 12/714,205 ("the '205 application"):
Claim 68: A method of treating or alleviating one or more symptoms of a nerve disorder mediated by nucleus pulposus in a mammal in need of such treatment comprising the step of administering a therapeutically effective amount of a TNF-a inhibitor to the mammal, wherein said TNF-a inhibitor is an antibody that blocks TNF-a activity, wherein the antibody is administered locally. (emphasis in opinion)
Claim 69: The method of claim 68, wherein the antibody is administered epidurally to the mammal. (emphasis in opinion)
The parties filed several motions before the Patent Trial and Appeal Board (the former Board of Patent Appeals and Interferences, which assumed jurisdiction over interferences under the AIA) (five motions by Tobinick, eleven by Olmarker). Relevant to this opinion, Senior Party Olmarker filed a motion to dismiss the interference on the grounds that Junior Party Tobinick did not have standing to provoke the interference because the specification of the '205 application did not provide an adequate written description of the claim element that the antibody is administered locally. Senior Party Olmarker argued that the '205 application described systemic but not local administration of a TNF-a inhibitor. This argument was based on the PTAB's construction that the term "locally administered" meant "administering a TNF-a inhibitor 'directly to the site where [the TNF-a inhibitor] is intended to act, that is, to the location where the nucleus pulposus is causing the symptoms of the nerve disorder,'" based on the intrinsic evidence of Senior Party Olmarker's U.S. Patent No. 7,708,995 ("the '995 patent"). Using this construction, the PTAB found that the '205 application lacked a written description satisfying the statute because "it did not sufficiently delineate between local and non-local administration." Specifically, the PTAB found that "local" administration as set forth in the ‘205 specification included administration of the medicament "near" the injury site, under circumstances where the medicament would diffuse to the region of injury. Under this construction the PTAB granted Senior Party Olmaker's motion and dismissed the interference.
The Federal Circuit affirmed the PTAB's claim construction but reversed its determination that the '205 application did not provide an adequate written description of the invention as recited in the counts. In an opinion by Judge Reyna, joined by Judges Lourie and Wallach, the Court affirmed the PTAB's claim construction based on the intrinsic evidence found in Senior Party Olmarker's '995 patent. The '995 patent sets forth explicitly the contrast between local and systemic administration, having an example where the TNF-a inhibitor is administered directly to the nucleus polposus from the injured spinal disk. No other method of local administration is disclosed in the '995 specification. In addition to this intrinsic evidence the PTAB also heard expert evidence from both parties, which included evidence from medical dictionaries as extrinsic evidence. Both parties agreed on the meaning of the term "local" and despite additional distinctions from Tobinick's expert the PTAB adopted Senior Party Olmarker's definition that local administration meant administration at the site where the TNF-a inhibitor would act. The Federal Circuit agreed that the term "administered locally" mean administration "directly to the site where it is intended to act, that is, to the location where the nucleus pulposus is causing the symptoms of the nerve disorder" and that this method of administration did not encompass "systemic administration away from the site where the TNF-a is intended to act."
Nevertheless, the Federal Circuit reversed the PTAB's determination that Junior Party Tobinick did not have standing to provoke the interference. According to the panel, disclosure in the '205 application of epidural TNF-a inhibitor administration provided the statutorily required adequate written description. The basis for the Court's decision to reverse is that the '205 specification "describes administering an inhibitor to the epidural space adjacent to a herniated disc, which is the location where nucleus pulposus causes nerve injury." The Court reversed, despite the nature of the question before it as a question of fact and as a consequence the deference the Court must grant factual decisions by the Patent and Trademark Office, pursuant to Dickerson v. Zurko and 5 U.S.C. § 706. The Court supported its determination that an adequate written description is provided by the '205 specification with express disclosure of local administration in the specification and the benefits thereof. According to the panel, "[t]he '205 application need only reasonably convey to one skilled in the art that Tobinick had possession of at least one embodiment that meets the Board's construction of local administration. The epidural injection technique is such an embodiment."
The case now returns to the PTAB for action on the parties other preliminary motions and a determination on the merits of which party was the first to invent methods for relieving nerve disorders associated with injury to spinal disks by local administration of a TNF-a inhibitor.
Tobinick v. Olmarker (Fed. Cir. 2014)
Panel: Circuit Judges Lourie, Reyna, and Wallach
Opinion by Circuit Judge Reyna