
Last month, in Celltrion, Inc. v. Genentech, Inc., District Judge Jeffrey S. White of the U.S. District Court for the Northern District of California granted two motions to dismiss filed by Defendants Genentech, Inc.; Hoffman La-Roche, Inc.; and City of Hope ("Genentech"), which sought to dismiss the first amended complaint filed by Plaintiffs Celltrion, Inc.; Celltrion Healthcare, Co. Ltd.; Teva Pharmaceuticals International GMGH; and Teva Pharmaceuticals USA, Inc. ("Celltrion"). Celltrion had initiated the dispute between the parties by filing complaints for declaratory judgment with respect to patents related to Genentech's Herceptin and Rituxan biologic drugs.
Both of the cases involve the Biologics Price Competition and Innovation Act of 2009 ("BPCIA"), which provides a regulatory approval pathway for biosimilar drugs. Under the BPCIA, a biologic licensed by the U.S. Food and Drug Administration is known as a reference product, and the entity that manufactures the reference product is known as the reference product sponsor ("RPS"). An entity that wishes to manufacture a biosimilar drug -- the biosimilar applicant (BA) -- may apply to the FDA for approval, and upon a showing that there are no "clinically meaningful differences" between the biosimilar drug and the biologic drug, can procure FDA approval for the biosimilar drug.
42 U.S.C. § 262(l) of the BPCIA prescribes a detailed mechanism for resolving infringement claims arising between the reference party sponsor and the biosimilar applicant, which is colloquially referred to as the "patent dance." A timeline showing a number of steps in that patent dance is depicted below:
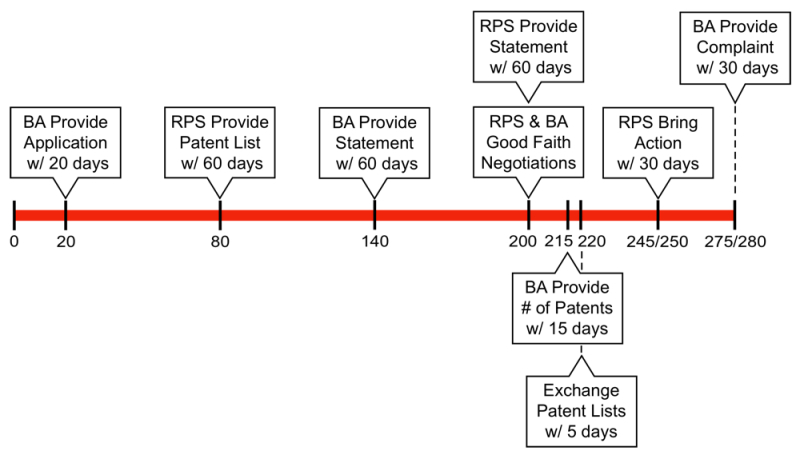 The two cases between Celltrion and Genentech relating to Herceptin and Rituxan involved §§ 262(l)(5)(A) and 262(l)(5)(B)(i), which specify the steps in the patent dance that take place after the biosimilar applicant and reference product sponsor engage in "good faith negotiations" to reach an agreement identifying the patents that will be the subject of patent infringement litigation (pursuant to § 262(l)(4)(A)). If the biosimilar applicant and reference product sponsor cannot agree on a list of patents to be litigated, the parties must simultaneously exchange lists of patents that each believes should be immediately litigated ("5(B) Lists"), and before that exchange takes place, the biosimilar applicant must identify the number of patents that it will identify on its own 5(B) List ("5(A) Number"). Pursuant to § 262(l)(9)(B), if a biosimilar applicant fails to serve its 5(A) Number or 5(B) List (or comply with several other steps), the applicant may not bring an action for declaratory judgment.
The two cases between Celltrion and Genentech relating to Herceptin and Rituxan involved §§ 262(l)(5)(A) and 262(l)(5)(B)(i), which specify the steps in the patent dance that take place after the biosimilar applicant and reference product sponsor engage in "good faith negotiations" to reach an agreement identifying the patents that will be the subject of patent infringement litigation (pursuant to § 262(l)(4)(A)). If the biosimilar applicant and reference product sponsor cannot agree on a list of patents to be litigated, the parties must simultaneously exchange lists of patents that each believes should be immediately litigated ("5(B) Lists"), and before that exchange takes place, the biosimilar applicant must identify the number of patents that it will identify on its own 5(B) List ("5(A) Number"). Pursuant to § 262(l)(9)(B), if a biosimilar applicant fails to serve its 5(A) Number or 5(B) List (or comply with several other steps), the applicant may not bring an action for declaratory judgment.
With respect to Herceptin, Celltrion had applied for FDA approval to market a biosimilar of that biologic drug called "Herzuma," and received notice that its application had been accepted by the FDA for review. When it came time for the parties to conduct good faith negotiations, Genentech proposed that the parties agree to litigate a discrete number (fewer than all) of the patents under discussion, and Celltrion responded by indicating that it wished to litigate a larger number of patents than Genentech's opening offer. Without providing its 5(A) Number or 5(B) List, Celltrion served a notice of commercial marketing on Genentech and then filed a declaratory judgment action against Genentech regarding the Herceptin patents it wished to litigate.
With respect to Rituxan, Celltrion had applied for FDA approval to market a biosimilar of that biologic drug called "Truxima," and received notice that its application had been accepted by the FDA for review. During good faith negotiations, Celltrion indicated that it wished to litigate all forty of the patents Genentech had placed on its § 262(l)(3)(A) List. As with Herceptin, Celltrion served a notice of commercial marketing on Genentech without providing its 5(A) Number or 5(B) List, and then filed a declaratory judgment action against Genentech regarding the Rituxan patents Celltrion wished to litigate.
Genentech responded to the declaratory judgment actions by filing motions to dismiss both suits for lack of subject matter jurisdiction, or alternatively, failure to state a claim. With respect to subject matter jurisdiction, the District Court noted that "Genentech has cited no 'clear statement' by Congress suggesting that Congress intended the BPCIA's requirements to be jurisdictional prerequisites," and therefore treated Genentech's motions as seeking dismissal for failure to state a claim under Federal Rule of Civil Procedure 12(b)(6).
In arguing against those motions, Celltrion contended that it may streamline its obligations under the BPCIA and satisfy several steps of the patent dance at once. In particular, Celltrion argued that it was absolved of the responsibility to comply with § 262(l)(5) because it told Genentech it "wished" to litigate all of the patents on Genentech's § 262(l)(3)(A) Disclosure, and that this statement both fulfilled its obligations to engage in "good faith negotiations" under § 262(l)(4) and made the exchange of the 5(A) Number and 5(B) Lists "redundant." The District Court, however, countered that "[t]his argument . . . improperly conflates Sections (l)(4) and (l)(5)," pointing out that "[t]he parties' obligations under Section (l)(5) only arise if the parties are unable to agree, after fifteen days of good faith negotiations, on a final and complete list of patents to litigate in Phase I." The Court concluded that "[g]iven the plain language of this provision, and the relationship between Sections (l)(4) and (l)(5) more generally[,] . . . no single statement or gesture can satisfy the requirements of both sections simultaneously."
With respect to the Rituxan complaint, Celltrion argued that that it was not obligated to offer its 5(A) Number or exchange 5(B) Lists because it filed its lawsuit nine days before the expiration of the fifteen-day period provided under § 262(l)(4) for good faith negotiation. The Court found this argument to be unpersuasive, noting that "[b]y this argument, Celltrion suggests that the filing of this declaratory judgment action was permissible because it skipped required statutory steps, where the non-occurrence of those statutory steps explicitly bars Celltrion from filing this action—an unpersuasive legal Catch-22." The Court stated that "Celltrion was obligated to complete all required procedures before filing this lawsuit, and it did not."
Celltrion's final argument was that the notices of commercial marketing it served for Herzuma and Truxima enabled it to file the declaratory judgment actions regardless of its compliance with other portions of the BPCIA. In particular, Celltrion contended that because a notice of commercial marketing lifts the ban on declaratory judgment actions described in § 262(l)(9)(A), a notice of commercial marketing should also lift §§ 262(l)(9)(B) and (C)'s prohibitions. The District Court, however, pointed out that the Central District of California had recently considered and rejected a similar argument in Amgen v. Genentech, Inc., 17-cv-7349-GHW, 2018 WL 910198 (C.D. Cal. Jan. 11, 2018). The Court reiterated that "a notice of commercial marketing only opens the door for an applicant to file a declaratory judgment action if the applicant complies with the rest of the statute."
"Because Celltrion did not complete its obligations under Section (l)(5)," the District Court determined that "Celltrion may not file actions for declaratory judgment with respect to the patents at issue," and therefore granted both of Genentech's motions to dismiss, while affording Celltrion leave to amend its complaints.
Celltrion, Inc. v. Genentech, Inc. (N.D. Cal. 2018)
Order Granting Defendants' Motions to Dimiss by District Judge White