
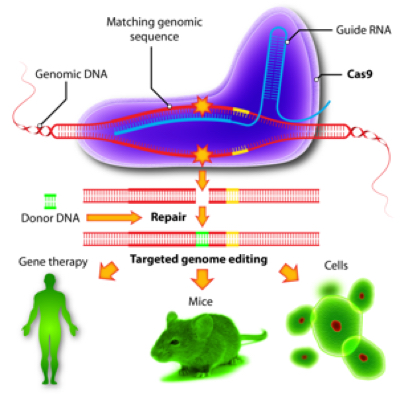
CRISPR (an acronym for Clustered Regularly lnterspaced Short Palindromic Repeats), which is part of a system for altering chromosomal sequences in situ in a cell in combination with a bacterially derived protein called Cas9, has been hailed as the "Breakthrough of the Year" for 2015, and rightfully so. Just as the discovery of bacterial restriction enzymes by Cohen and Boyer in 1972 provided the ability to dissect DNA at specific sites in the DNA sequence, CRISPR provides a mechanism for inserting or deleting specific DNA sequences using CRSPR-associate targeting RNAs and the Cas9 RNA-guided DNA endonuclease enzyme. It provides for the first time the type of specificity for altering DNA that the polymerase chain reaction (PCR) provided a generation ago, as illustrated by this schematic:
 Given the commercial potential of this method, patenting is an obvious concern and, as it turns out, more than one group of inventors has filed patent applications on the reagents, methods, and cells produced or used to produce CRISPR modifications. Because these applications were filed prior to March 16, 2013, the inventor(s) who first invented this invention have the right to patent it, and disputes regarding who was the first to invent are resolved using a procedure called an interference.
Given the commercial potential of this method, patenting is an obvious concern and, as it turns out, more than one group of inventors has filed patent applications on the reagents, methods, and cells produced or used to produce CRISPR modifications. Because these applications were filed prior to March 16, 2013, the inventor(s) who first invented this invention have the right to patent it, and disputes regarding who was the first to invent are resolved using a procedure called an interference.
In this case, on January 11, 2016, the U.S. Patent and Trademark declared Interference No. 106,048 under the provisions of 37 C.F.R. § 41.203(b), naming Feng Zhang and his colleagues, the named inventor of the Broad Institute/MIT's patents, are the Junior Party, and Jennifer Doudna and her colleagues at UC/Berkeley are Senior Party (see Declaration). These designations stem from which party was the first to file a patent application and are important, because (without evidence to the contrary) the PTO considers the Senior Party to be the presumptive inventor (and thus the burden to establish prior right based on earlier invention is borne by the Junior Party).
Paradoxically, the Broad Institute/MIT inventors have obtained a number of patents even though the Berkeley inventors have the earliest filing date and no issued patents (which is important because an interference cannot resolve the dispute between patents granted on the same invention). This is because the Broad availed itself of priority examination ("fast track") provisions of the law. In fact, the Broad attempted to provoke an interference with the Berkeley patents, albeit involving a fewer number of patents and claims than are involved in this interference. The Broad patents (and their involved claims, which constitute all of the granted claims of all of the Broad patents) are:
• Patent 8,697,359 – claims 1-20
• Patent 8,771,945 – claims 1-29
• Patent 8,795,965 – claims 1-30
• Patent 8,865,406 – claims 1-30
• Patent 8,871,445 – claims 1-30
• Patent 8,889,356 – claims 1-30
• Patent 8,895,308 – claims 1-30
• Patent 8,906,616 – claims 1-30
• Patent 8,932,814 – claims 1-30
• Patent 8,945,839 – claims 1-28
• Patent 8,993,233 – claims 1-43
• Patent 8,999,641 – claims 1-28*,
while only one pending application from the Berkeley group is involved:
• US 2014-0068797 A1 - claims 165, 200, 202-218, 220-222 and 224-247.
Representative claims of each party are as follows:
The Broad/MIT:
1. An engineered, non-naturally occurring CRISPR-Cas system comprising one or more vectors comprising:
a) a first regulatory element operable in a eukaryotic cell operably linked to at least one nucleotide sequence encoding a CRISPR-Cas system guide RNA that hybridizes with a target sequence of a DNA molecule in a eukaryotic cell that contains the DNA molecule, wherein the DNA molecule encodes and the eukaryotic cell expresses at least one gene product, and
b) a second regulatory element operable in a eukaryotic cell operably linked to a nucleotide sequence encoding a Type-II Cas9 protein,
wherein components (a) and (b) are located on same or different vectors of the system, whereby the guide RNA targets and hybridizes with the target sequence and the Cas9 protein cleaves the DNA molecule, whereby expression of the at least one gene product is altered; and, wherein the Cas9 protein and the guide RNA do not naturally occur together.
5. A method of altering expression of at least one gene product comprising introducing into a eukaryotic cell containing and expressing a DNA molecule having a target sequence and encoding the gene product an engineered, non-naturally occurring Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)--CRISPR associated (Cas) (CRISPR-Cas) system comprising one or more vectors comprising:
a) a first regulatory element operable in a eukaryotic cell operably linked to at least one nucleotide sequence encoding a CRISPR-Cas system guide RNA that hybridizes with the target sequence, and
b) a second regulatory element operable in a eukaryotic cell operably linked to a nucleotide sequence encoding a Type-II Cas9 protein,
wherein components (a) and (b) are located on same or different vectors of the system, whereby the guide RNA targets the target sequence and the Cas9 protein cleaves the DNA molecule, whereby expression of the at least one gene product is altered; and, wherein the Cas9 protein and the guide RNA do not naturally occur together.
6. A CRISPR-Cas system-mediated genome editing method comprising introducing into a eukaryotic cell containing and expressing a DNA molecule having a target sequence and encoding at least one gene product an engineered, non-naturally occurring CRISPR-Cas system comprising one or more vectors comprising:
a) a first regulatory element operable in a eukaryotic cell operably linked to at least one nucleotide sequence encoding a CRISPR-Cas system guide RNA that hybridizes with the target sequence, and
b) a second regulatory element operable in a eukaryotic cell operably linked to a nucleotide sequence encoding a Type-II Cas9 protein,
wherein components (a) and (b) are located on same or different vectors of the system, whereby expression of the at least one gene product is altered through the CRISPR-Cas system acting as to the DNA molecule comprising the guide RNA directing sequence-specific binding of the CRISPR-Cas system, whereby there is genome editing; and, wherein the Cas9 protein and the guide RNA do not naturally occur together.
Berkeley:
165. A method of cleaving a nucleic acid comprising contacting a target DNA molecule having a target sequence with an engineered and/or non-naturally-occurring Type II Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-CRISPR associated (Cas) (CRISPR-Cas) system comprising
a) a Cas9 protein; and
b) a single molecule DNA-targeting RNA comprising
i) a targeter-RNA that hybridizes with the target sequence, and
ii) an activator-RNA that hybridizes with the targeter-RNA to form a double-stranded RNA duplex of a protein-binding segment,
wherein the activator-RNA and the targeter-RNA are covalently linked to one another with intervening nucleotides,
wherein the single molecule DNA-targeting RNA forms a complex with the Cas9protein,
whereby the single molecule DNA-targeting RNA targets the target sequence, and the Cas9 protein cleaves the target DNA molecule.
203. An engineered and/or non-naturally occurring Type II Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-CRISPR associated (Cas) (CRISPR-Cas) system comprising
a) a Cas9 protein, or a nucleic acid comprising a nucleotide sequence encoding said Cas9 protein; and
b) a single molecule DNA-targeting RNA, or a nucleic acid comprising a nucleotide sequence encoding said single molecule DNA-targeting RNA;
wherein the single molecule DNA-targeting RNA comprises:
i) a targeter-RNA that is capable of hybridizing with a target sequence in a target DNA molecule, and
ii) an activator-RNA that is capable of hybridizing with the targeter-RNA to form a double-stranded RNA duplex of a protein-binding segment,
wherein the activator-RNA and the targeter-RNA are covalently linked to one another with intervening nucleotides; and
wherein the single molecule DNA-targeting RNA is capable of forming a complex with the Cas9 protein, thereby targeting the Cas9 protein to the target DNA molecule,
whereby said system is capable of cleaving or editing the target DNA molecule or modulating transcription of at least one gene encoded by the target DNA molecule.
224. A Type II Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-CRISPR associated (Cas) (CRISPR-Cas) system comprising:
a Cas9 protein; and
a single molecule DNA-targeting RNA, or a nucleic acid comprising a nucleotide sequence encoding said single molecule DNA-targeting RNA,
wherein the single molecule DNA-targeting RNA comprises:
i) a targeter-RNA that is capable of hybridizing with a target sequence in a target DNA molecule, and
ii) an activator-RNA that is capable of hybridizing with the targeter-RNA to form a double-stranded duplex of a protein-binding segment,
wherein i) and ii) are arranged in a 5' to 3' orientation and are covalently linked to one another with intervening nucleotides;
wherein the single molecule DNA-targeting RNA is capable of forming a complex with the Cas9 protein and hybridization of the targeter-RNA to the target sequence is capable of targeting the Cas9 protein to the target DNA molecule, and
wherein the single molecule DNA-targeting RNA comprises one or more sequence modifications compared to a sequence of a corresponding wild type tracrRNA and/or crRNA.
In an interference, the PTO establishes one or more "counts," which is a phantom claim that encompasses the interfering subject matter. The parties provide evidence of conception of at least one embodiment falling within the scope of the count and reduction to practice (actual or constructive, i.e., by filing a patent application having an enabling disclosure of said one embodiment). While there are several complicated scenarios that can arise in an interference, generally a party that conceived first and reduced to practice first, and did not abandon, suppress or conceal the invention, will prevail. The sole Count in the CRISPR interference reads as follows:
Count 1
A method, in a eukaryotic cell, of cleaving or editing a target DNA molecule or modulating transcription of at least one gene encoded thereon, the method comprising:
contacting, in a eukaryotic cell, a target DNA molecule having a target sequence with an engineered and/or non-naturally-occurring Type II Clustered Regularly lnterspaced Short Palindromic Repeats (CRISPR)-CRISPR associated (Cas) (CRISPR-Cas) system comprising:
a) a DNA-targeting RNA comprising
i) a targeter-RNA or guide sequence that hybridizes with the target sequence, and
ii) an activator-RNA or tracr sequence that hybridizes with the targeter-RNA to form a double-stranded RNA duplex of a protein-binding segment, and
b) a Cas9 protein,
wherein the DNA-targeting RNA forms a complex with the Cas9 protein, thereby targeting the Cas9 protein to the target DNA molecule, whereby said target DNA molecule is cleaved or edited or transcription of at least one gene encoded by the target DNA molecule is modulated.
The interference will proceed in two stages. The first stage involves the parties presenting motions that can modify the count, have certain claims declared outside the scope of the count (or vice versa), and ask for a finding that the claims are invalid under any of the provisions of the patent statute. If these motions are not decided in a way that would disqualify one or both parties, then the interference will move to a second stage, where the Junior Party (The Broad) will present its proofs of conception and reduction to practice and the Senior Party will be permitted to oppose. The Senior Party is under no obligation to present proofs earlier than its earliest filing date unless the Junior Party evinces evidence of (at least) earlier conception. In practice, the parties can both be expected to submit their evidence.
The parties can also settle the inference privately, with the losing party filing a Concession of Priority and the prevailing party (usually) granting a license to the loser; such settlement agreements are kept confidential but must be filed. Otherwise, everything else in an interference is public information and can be found under the Patent Trial and Appeal Board (PTAB) on the Office's PAIR website. Office rules mandate the times for these two stages and an interference is usually concluded with a decision (if not earlier settled) within 36 months of the Declaration.
* The Broad/MIT also have several pending applications, including US 2014-0186919 A1; US 2014-0179770 A1; US 2014-0242664 A1; US 2014-0310830 A1; US 2014-0189896 A1; US 2014-0242699 A1; US 2014-0357530 A1; US 2015-0020223 A1; and US 2015-0079681 A1. Should allowable claims be presented in any of these applications such claims may become involved in this interference.