Recently, the Federal Circuit issued its holding in a case dealing with asserting claims of an extended patent covering administration of dimethyl fumarate formulations to treat multiple sclerosis (MS). Dimethyl fumarate (DMF) is the U.S. Food and Drug Administration (FDA) approved ingredient in the multiple sclerosis drug Tecfidera®. Tecfidera had more than $4 billion in sales in 2019.
Biogen International GmbH (Biogen), the manufacturer of Tecfidera, holds U.S. Patent Number 7,619,001 (the '001 patent). The '001 patent contains method of treatment claims that recite treating MS by administering, to a MS patient, a formulation containing DMF, monomethyl fumarate (MMF), or a combination of these.
The '001 patent was originally set to expire in April 2018. However, Biogen filed an application for patent term extension (PTE), and was successful in extended the term of the '001 patent by 811 days, or about 2.2 years. Each day of Tecfidera market exclusivity is worth approximately $11 million.
Because Tecfidera was first commercially marketed after approval under section 505(b)(1) of the Federal Food, Drug, and Cosmetic Act, Tecfidera was granted five years of data exclusivity. Tecfidera was approved by the FDA in March 27, 2013, and its five years of exclusivity expired about two years ago.
In 2018, after Tecfidera's five-year period of data exclusivity had expired, Banner Life Sciences LLC, or Banner, submitted a paper new drug application, or paper NDA, to market a twice daily pill containing MMF for the treatment of MS. DMF is a diester molecule (ester groups shown in blue), and MMF is a monoester molecule that also contains a carboxylic acid group (shown in red):
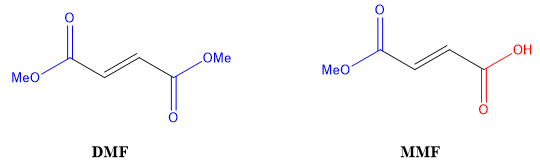
In December 2018, Biogen asserted the '001 patent against Banner. Banner argued the PTE statute "limits the scope of the '001 patent's extension methods of using DMF, its salts or its esters, and that MMF is none of those things." The trial court agreed and held for Banner, rendering a judgment of non-infringement. Biogen appealed.
The PTE statute holds, in part that PTE, "in the case of a patent which claims a method of using a product, be limited to any use claimed by the patent and approved for the product." The PTE statute also defines the term product, in part, to mean "drug product." Finally, the PTE statute defines the term "drug product" in part to mean the "active ingredient of … a new drug … including any salt or ester of the active ingredient … as a single entity." (Emphasis added.)
The appeals court agreed with Banner. The appeals court reasoned, in part, that "'[a]ctive ingredient' is a term of art, defined by the FDA as 'any component that is intended to furnish pharmacological activity or other direct effect'" and, citing to an earlier case, that the active ingredient "must be present in the drug product when administered." (Emphasis added.)
The appeals court reasoned:
The active ingredient of a given drug product is defined by what is approved and is specified on the drug's label … MMF is not the approved product, nor is it specified as the active ingredient on the Tecfidera® label. Esters are included in the statutory definition of what can be extended, but MMF is the de-esterified form of DMF, not an ester of DMF. Thus, it is not the same product under § 156(f) and does not fall within the scope of the '001 patent's term extension … (Emphasis added.)
Put succinctly, for purposes of the PTE statute, the appeals court reasoned that: product = drug product = active ingredient including any salt or ester of the active ingredient which must be present in the drug product when administered. Because MMF:
- was not present in Tecfidera when administered;
- was not specified as the active ingredient in Tecfidera's drug label; and
- was not a salt or ester of the active ingredient (DMF) in Tecfidera;
the appeals court held that the claims of the '001 patent, under the PTE statute, did not extend to cover MMF, affirming the decision of the trial court.
Our Comments
This case serves as a caution that courts may interpret the scope of claims differently depending on whether a patent is pending during its normal term, versus when the patent is in force during a period of PTE. And because MMF is a metabolite of DMF, the case may be applicable for patent holders attempting to assert certain prodrug claims against a competitor coming to market with a metabolite active ingredient.
If the '001 patent was in force and asserted under its non-extended patent term, the case outcome could have been different. Claim 1 of the '001 patent, which recites (in part) administering a pharmaceutical formulation containing MMF to a patient to treat MS. Claim 1 of the '001 patent, when asserted for its full scope, could arguably have been found to be infringed by Banner's MMF containing drug formulation which is indicated for treating MS.
But the claim scope coverage provided by claim 1 of the '001 patent was curtailed by statute when the '001 patent entered its PTE phase. This case provides a caution to drug developers who make certain prodrugs (e.g., esters), and seek FDA approval of the prodrugs, that the scope of their patent claims could be curtailed for the period that any patent containing these claims remains in force solely under PTE.
The case also highlights the non-commutative nature of PTE when the approved drug contains a carboxylic acid and the generic or 505(b)(2) competitor's drug is an ester of the acid, versus when the approved drug contains an ester of a carboxylic acid, and the generic or 505(b)(2) competitor's drug contains a carboxylic acid in place of the ester. For example, if an approved drug contains carboxylic acid; a generic or 505(b)(2) competitor attempts to come to market with an ester (prodrug) of the carboxylic acid of the approved drug; the branded drug manufacturer has patent claims that include an ester of the approved drug; and a patent containing these claims is extended; arguably the generic or 505(b)(2) competitor could be found to infringe the claims of the extended patent. But as the holding of this case illustrates, the converse may not be true.
Another issue raised by the case is the choice of molecule to develop. If a drug company faces a choice to develop a drug candidate containing, for example, a carboxylic acid or an ester of the carboxylic acid, from a PTE perspective, development of the drug candidate containing the carboxylic acid (or its salt) may be a better choice.
Finally, the case raises additional considerations. For example, would the outcome have been different if MMF was on Tecfidera's label and package insert? And why did Biogen come to market with DMF if it knew MMF was the active metabolite and could be formulated into a drug formulation?