 Last week, the Generic Pharmaceutical Association (GPhA) released a statement regarding a California bill (SB-598) that the GPhA asserted would "introduce[] burdensome provisions that could raise costs and limit patient access to more affordable biosimilar medicines." The bill, introduced in February and now before Governor Jerry Brown, would authorize a pharmacist to select a biosimilar when filling a prescription order for a prescribed biological product, provided that the prescriber did not personally indicate "Do not substitute." The legislation would also require that the substitution of a biosimilar be communicated to the patient, and that the pharmacy notify the prescriber of the substitution or enter the substitution into a patient record within five business days of the selection.
Last week, the Generic Pharmaceutical Association (GPhA) released a statement regarding a California bill (SB-598) that the GPhA asserted would "introduce[] burdensome provisions that could raise costs and limit patient access to more affordable biosimilar medicines." The bill, introduced in February and now before Governor Jerry Brown, would authorize a pharmacist to select a biosimilar when filling a prescription order for a prescribed biological product, provided that the prescriber did not personally indicate "Do not substitute." The legislation would also require that the substitution of a biosimilar be communicated to the patient, and that the pharmacy notify the prescriber of the substitution or enter the substitution into a patient record within five business days of the selection.
The GPhA noted that a group of more than thirty organizations, including CalPERS, AARP, California Pharmacists Association, California Association of Health Plans, nine state labor unions, Kaiser Permanente and some retail pharmacies, "oppose the burdensome notification provisions in SB 598 and call upon Governor Brown to veto the legislation." The GPhA also noted that similar legislation has been rejected in ten of the eighteen states where it has been considered and has only been passed with "significant amendments" in three states and with "Amgen and Genentech-backed provisions intact in only one state (North Dakota)."
In opposing the California bill, the GPhA points to a report from Express Scripts, which determined that the potential 10-year saving to patients and payers in California from the introduction of biosimilars on eleven biologics that are protected by U.S. patents that have expired or will expire in the near future unless new patents are granted would be approximately $27.6 billion. The report, entitled "Ten-Year Potential Savings from Biosimilars in California," and authored by Dr. Sharon Glave Frazee, Dr. Susan Garavaglia, Jonah Houts, and Dr. Steve Miller, looked at eleven brand biologic drugs that were considered to be good candidates for the introduction of biosimilars over the next ten years. The eleven biologics are listed in Table 1 of the report:
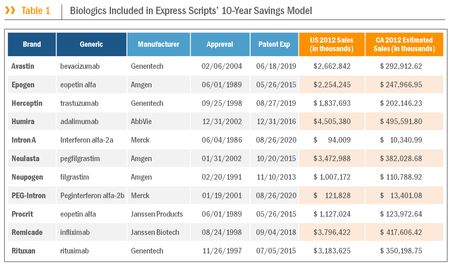 Noting that "[t]he projected unrealized saving is significant," the report states that the potential savings "warrant[] regulatory and legislative consideration at both federal and state levels to improve the pathway for biosimilar evaluation and reject industry requests to enact legislative barriers to interchangeable biosimilar substitution."
Noting that "[t]he projected unrealized saving is significant," the report states that the potential savings "warrant[] regulatory and legislative consideration at both federal and state levels to improve the pathway for biosimilar evaluation and reject industry requests to enact legislative barriers to interchangeable biosimilar substitution."
The GPhA indicated that the report "reinforces the importance of improving the pathway for biosimilar evaluation and rejecting requests to enact legislative barriers to interchangeable biosimilar substitution," and "bolsters the argument that adding hurdles to the biosimilar pathway only can do harm to patients, payers and the general public."