
By Cambria Alpha-Cobb* and Anthony D. Sabatelli** --
Late last year, the Tufts Center for the Study of Drug Development (CSDD) released a number that would cause jaws to drop all around the healthcare world. The estimated cost for developing a new drug was an astounding $2.558 billion. Can we believe this number? If this high price is the new reality, how can drug developers protect their investment? More importantly, is your patent portfolio up to the challenge of protecting this huge investment?
As the cost of bringing a new drug to market soars, it is becoming ever more important for drug developers to ensure that their development efforts are adequately protected. Even though the FDA awards up to five years of regulatory exclusivity upon approval of a new drug compound (twelve years for a biologic), this period is often far too short for a drug innovator to recoup their development costs. Therefore, it is essential that drug developers construct a well thought out patent strategy. This strategy should fully consider managing the product life cycle for a new drug from the earliest development efforts through to the mature stages of marketing when the drug is facing competition from generic manufactures.
The process of building a patent portfolio strategic enough to protect a drug franchise can be very involved and expensive. For example, a single patent that is filed, prosecuted, and maintained across a reasonably broad group of the major market countries of the world can easily cost over a million dollars. However, such an investment still only represents less than 1 percent of the total drug development costs according to the Tufts' figure -- clearly a wise investment.
To fully comprehend the impact and importance of this study, it is necessary to understand where it comes from. The Tufts CSDD is a non-profit, multi-disciplinary research group associated with the academic institute of Tufts University. This research group has been gathering and analyzing a variety of information around the quality and efficiency of drug R&D since the 1970s. The CSDD studies are expectantly waited for and used by public policy makers and drugs developers, among others. This recent study randomly selected drugs tested in humans and analyzed the cost of their development within a designated time period. The CSDD is in a unique position to provide this sort of analysis as it has used comparable methodology to record and analyze components of drug development for forty years. This mass accumulation of similarly acquired information has allowed for analogous studies of the development process and how it has transformed over time.
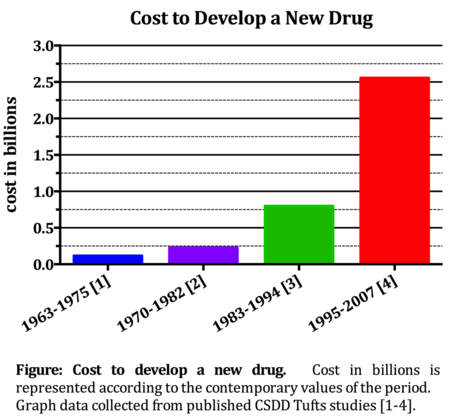
This most recent cost estimate included 106 investigational new drugs from 10 organizations around the world that were tested in humans between 1995 and 2007. This $2.558 billion figure was calculated from an average $1.4 billion in out-of-pocket costs and $1.2 billion in returns that investors forego during the development and approval process. The inclusion of post approval development, involving new indication testing, formulations, and dosage strengths, adds another $312 million, bringing the grand total life-cycle cost of developing a new drug to nearly $3 billion.
As stated above, in the U.S. and some other countries, drug developers can hope to recover some of this cost via the exclusivity period granted for the development and approval of a new drug. But is this enough? Only 20% of approved medicines generate revenues that exceed the average R&D investment (Michael Rosenblatt). Without adequate patent protection, few can hope to recover these costs. Clearly, a solid, well-executed patent portfolio is needed to augment this regulatory exclusivity. Many would argue that without this system of both patent and regulatory exclusivity, innovation would slow as this high investment cost is enough to disincentivize even the best ideas.
Can we believe this number?
Upon the announcement of this ten-digit number, the researchers and their results were quickly criticized, being interpreted as a ploy by pharmaceutical companies to justify the high costs of new drugs. The fact that 40% of the CSDD's funding comes from pharmaceutical and biotechnology firms led many to question the reality behind this argument and to do their own fact checking. However, Bruce Booth, a Forbes contributor and partner at Atlas Venture, published his own estimate, coming up with a $2.5 billion number, factoring in rates of program success, timelines for each phase of R&D, and direct spend on projects. As other such independent estimates have emerged, citing similar costs, the skepticism around this study has diminished and the reality of the number is sinking in.
In order to understand this reality, we need to examine the possible explanations as to why it is so pricey. We plotted the cost to develop a new drug as reported in 1979, 1991, 2003 and now in 2014 CSDD Tuft's studies to really demonstrate the almost exponential increase over the past decades (see Figure). The cost to develop a new drug has more than doubled since the last Tufts analysis, published in 2003, where the cost was equal to $1.044 billion in 2013 dollars (DiMasi 2003). As an explanation for this dramatic increase, the Tufts study cites a general trend in increasing costs due to greater out-of-pocket costs. Included in these costs are the increases in clinical trial complexity and size, higher costs of inputs from the medical sector, a greater focus on targeting chronic and degenerative diseases and increased testing required by insurers for comparative effectiveness data, among others. Additionally, the CSDD cites higher failure rates in the human trials. This means that more drugs must be screened, more trials must be run, and more capital must be spent in order to bring just one drug to market. The $2.6 billion estimate reflects, as it should, the cost of both successful and unsuccessful R&D projects. Therefore, the estimate reflects even those drugs with failed trials whose development was halted early, and whose developing company recouped little or none of the development investment.
How should we react to this number?
Regardless of the exact number of zeros behind the estimate, the conclusion is the same: the cost to bring a drug to market is very large. Much of this study's criticism comes from the accusation that pharmaceutical researchers and manufacturers will use this study to justify the high cost of drugs. This argument, of course, assumes that the price of drugs is directly linked to the costs incurred during research and development. As the former president of Pfizer Global Research and Development, John LaMattina states, "pricing should be based not on R&D costs but on the value a drug delivers to patients."
LaMattina goes on to argue that the real value of the Tufts study lies in the increasing of public awareness to the long, expensive, and high-risk endeavor that drug-development encompasses. A common argument is that patent monopolies control and unfairly manipulate the cost of drugs that would be relatively cheap in the free market. This study however is direct evidence in contrary to that argument. How could one hope to see a cheaper drug on the market when the R&D costs are so high?
No company would take on the gamble of the drug development process were it not for the exclusivity granted to the developer upon approval by the FDA. The U.S. patent system provides additional incentive to develop, innovate, and further the benefits of modern medicine, even when the costs are high. Painting an accurate estimation of drug R&D not only leads to a public awareness of the risks involved, but also emphasizes why patenting strategies are so important for drug developers.
As we previously mentioned, the cost for protection also pales in comparison to the development costs. This protection is essential for redemption of a significant portion of the development costs.
Knowing the high cost of drug development probably will not and cannot lead to changes in the R&D process or costs. If drug researchers and manufacturers want to lower the cost, the solution is to develop faster and cheaper! But that is easier said than done. This exposure of the high cost of drug development is a wake up call and reminder to the world that new, life-saving drugs are expensive to develop. Even if this study cannot bring cheaper drugs to our doorstep, it brings into the public eye the uncertainty of drug development and better describes the risks, capital, and time behind every bottle of pills that we take for granted.
* Dr. Cambria Alpha-Cobb is a Technology Specialist at Dilworth IP
** Dr. Sabatelli is a Partner with Dilworth IP
[1] Hansen, R.W., 1979. The pharmaceutical development process: estimates of current development costs and times and the effects of regulatory changes. In: Chien, R.I. (Ed.), Issues in Pharmaceutical Economics. Lexington Books, Lexington, MA, pp. 151–187.
[2] DiMasi, J.A., Hansen, R.W., Grabowski, H.G., Lasagna, L., 1991. Cost of innovation in the pharmaceutical industry. Journal of Health Economics 10, 107–142. http://www.ncbi.nlm.nih.gov/pubmed/10113009
[3] DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. Journal of Health Economics 2003;22(2):151-85. http://www.ncbi.nlm.nih.gov/pubmed/12606142
[4] http://csdd.tufts.edu/news/complete_story/pr_tufts_csdd_2014_cost_study