
[authors: David Puleo* and Anthony D. Sabatelli**]
Introduction
Cancer immunotherapy or immuno-oncology (I-O) has gone mainstream. You may have heard about these topics in the media. Along with having surgery and radiation therapy, Former President Jimmy Carter was treated with the monoclonal antibody pembrolizumab (Keytruda®) that stimulated his immune system to fend or fight off melanoma that had metastasized to his brain. His remarkable response has highlighted the power of immunotherapy. After his son Beau succumbed to brain cancer, Vice President Joe Biden helped spearhead the Cancer Moonshot, a national initiative to cure cancer, one of the main goals of which is furthering immunotherapy research. The White House has dedicated $1 Billion to this initiative. This article will introduce you to immunotherapy and how it is now beginning to intersect with the microbiome. Recent patent filings suggest that intellectual property protection will be an important part of the research efforts in this field. Bolded patent documents are further summarized in the table at the end of this installment.
What is Immunotherapy?
Put simply, immunotherapy harnesses the body's own immune system to fight cancer. Immunotherapy refers to those pharmacological agents that stimulate an immune response and, further, have been used to combat various forms of cancer. The most widely recognized immunotherapeutic class is the immune checkpoint proteins, which are a set of proteins that act as either stimulators or inhibitors of the immune system. The pharmaceutical industry has begun targeting the inhibitory immune checkpoint proteins. By dampening or attenuating the "off-switch", an immune response is enhanced. The most well-known checkpoint targets are cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), programmed cell death 1 (PD-1), and programmed death ligand 1 (PD-L1). A number of FDA-approved biologics, termed immune checkpoint inhibitors or checkpoint blockades, are on the market targeting these receptors, including ipilimumab (Yervoy®, Bristol-Myers Squibb, anti-CTLA-4), pembrolizumab (Keytruda®, Merck, anti-PD-1), and nivolumab (Opdivo®, Bristol-Myers Squibb, anti-PD-1), and have been used to treat a number of indications, including melanoma.
One glaring problem or challenge associated with immunotherapy is that the majority (60-80%) of patients, aptly termed "non-responders", do not respond to single agent therapy. Most non-responders lack sufficient amount of those immune cells, known as tumor-infiltrating lymphocytes (TILs), that are necessary to mobilize an anti-cancer immune response. Given this poor patient response and unwanted side effects, such as immunotherapy-induced colitis, due to single agent immune checkpoint inhibitor treatment, efforts have turned toward administering these biologics and other immunotherapeutics in combination. For instance, Bristol-Meyers Squibb's (BMS) Opdivo®-Yervoy® treatment is the first combination therapy approved for metastatic melanoma. Also, Opdivo® recently fell short to make its endpoint in a clinical trial as a single agent for frontline non-small cell lung cancer (NSCLC) therapy. This clinical trial was further highlighted at the recent European Society of Medical Oncology 2016 Congress, where Merck's Keytruda® was viewed as the heavy hitter (and is a serious contender for frontline NSCLC therapy). Given the recent Opdivo® results, BMS is looking to combine the biologic with other therapies, including Nektar Therapeutics' TIL-inducing NKTR-214. In theory, this combination should decrease the percentage of Opdivo® non-responders for a number of indications.
Microbiome-based Therapeutics as Combination Therapy
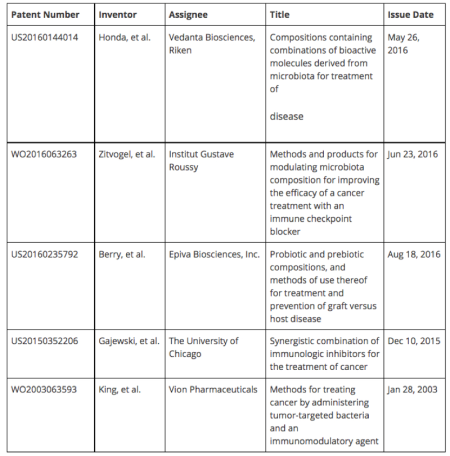
Two back-to-back articles in a 2015 issue of Science (350, 1079-1089) addressed how the microbiome could be a game-changer in I-O. Both articles highlighted the importance of commensal gut microbiota in the efficacy of single agent immunotherapy. The first paper was published by Laurence Zitvogel, Director of INSERM and Group Leader of Tumor Immunology and Anti-cancer Immunotherapy at Institut Gustave Roussy in France. His group had previously determined that the efficacy of the chemotherapeutic cyclophosphamide was dependent on the microbiome composition [Science 342, 971-976 (2013)]. They extended this work to immunotherapy and found that the bacterium Bacteroides fragilis improved the antitumor efficacy of an anti-CTLA-4 antibody. Given these intriguing results, Zitvogel filed patent application WO2016063263 for the administration of Bacteroides supplements to patients on an anti-CTLA-4 mAb, e.g. Yervoy®. Zitvogel and Institut Gustave Roussy are now partnering with the French biotech, Enterome Biosciences to further this research.
The second paper was published by Thomas Gajewski from The University of Chicago. His group found that Bifidobacterium improved the antitumor efficacy of an anti-PD-L1 antibody in a mouse model of melanoma. Although Gajewski filed patent application US20150352206 directed to specific immunotherapeutic combination therapies, this patent does not mention manipulation of the microbiome. Gajewski has licensed his work to Evelo Biosciences, known for their OncobioticTM Platform that was summarized in Part VI of the Series. Evelo recently merged with Epiva Biosciences; the new company retains the name Evelo. Both owned by Flagship Ventures, these companies were targeting the microbiome but doing so in different ways. Whereas Evelo was trying to treat cancer, Epiva was trying to treat autoimmune and inflammatory diseases (patent application US20160235792 summarizes Epiva's efforts). Evelo is using its trademarked platform to identify "cancer-associated bacteria" and further hopes to develop "onco-microbials" and "immuno-microbials", microbiome modulators for the treatment of cancer (namely lung, skin, prostate, and colon cancers) and autoimmune diseases, respectively.
The Future: Combinations of Microbiome Modulators and Checkpoint Inhibitors?
Since the previous studies by Zitvogel and Gajewski were performed in mice, scientists have yet to determine whether this translates to humans as well. It is unclear whether microbiome modulators could be used in combination with or would have any effect on other forms of immunotherapy, such as IDO inhibitors or CAR-T. However, it is evident that the interplay between the microbiome and targeted therapies is not straightforward and only adds a further layer of complexity to personalized medicine. Utilizing the microbiome-based diagnostics highlighted in Part VI of the Series, it is possible to identify and further compare the microbiomes of responder and non-responder patients being treated with similar immunotherapies. In doing so, one can further correlate a patient's microbiome composition with the clinical efficacy of immunotherapeutics. This data could be used to supplement non-responders with missing or deficient microbiome components to make their treatments more efficacious. Thus, combination therapies of immunotherapeutics with microbiome modulators are likely the way of the future. Several patent applications reflect this trend. An early patent application WO2003063593 from the defunct Vion Pharmaceuticals refers to using combinations of an immunomodulatory agent, such as anti-CD8 antibody, cyclosporine A, or methotrexate, with tumor-targeted bacteria in order to reduce solid tumor growth. US20160144014 discloses combinations of Clostridia and an immune modulator. Within the next several years, the terms "oncobiotics" and "immunobiotics" will likely become part of everyday scientific vernacular.
* David Puleo is a Ph.D. Candidate in the Pharmacology Department at Yale University. Prior to attending Yale, David graduated from Boston College with a B.S. in Biochemistry, after which he worked for two years in the Center for Proteomic Chemistry at Novartis Institutes for BioMedical Research in Cambridge, MA.
** Dr. Sabatelli is a Partner with Dilworth IP