
The Federal Circuit continues its explication of the law of obviousness post-KSR Int'l. v. Teleflex Inc. (and Judge Pauline Newman continues to disagree with her brethren in some regards) in a decision handed down last Friday, in Merck Sharp & Dohme Corp. v. Hospira, Inc.
The case arose in an ANDA litigation between innovator Merck and generic drug manufacturer Hospira over the antibiotic ertapenem, sold by Merck under the brand name Invanz®. The drug, known to be chemically unstable, has the following structure:
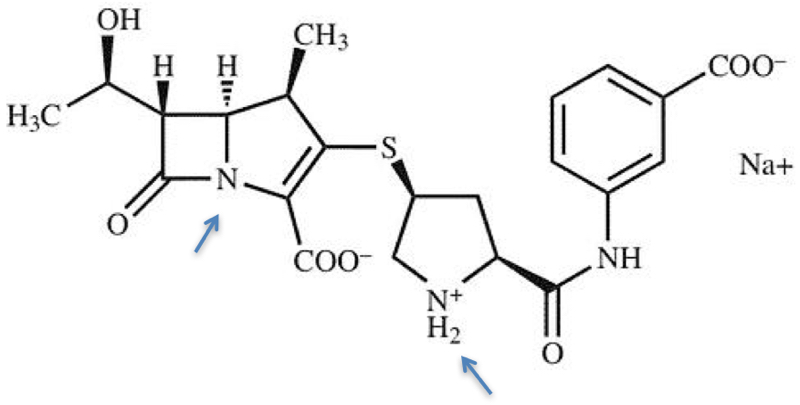
The molecule's instability arises from two aspects and from two different chemical reactions: hydrolysis of the beta-lactam nitrogen indicated by the arrow on the left, and dimerization of the pyrrolidine nitrogen on the right. The prior art taught that the dimerization reaction could be inhibited and the molecule stabilized by forming a carbon dioxide adduct after reaction with carbon dioxide under basic conditions.
The claimed invention is directed to minimizing both dimerization and hydrolysis, resulting in the stabilized form of ertapenem sold by Merck as its Invanz® product. One of the patents-in-suit (and the subject of this appeal) is U.S. Patent No. 6,486,150; claim 21 is representative of the asserted claims:
21. A process for preparing a final formulation product of a compound of formula Ia,
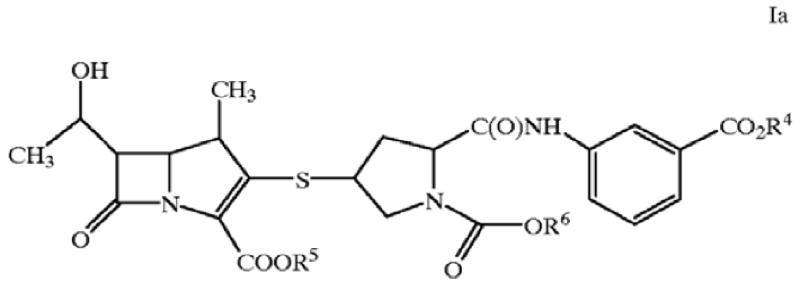
or its pharmaceutically acceptable salt, or hydrates wherein, R4, R5, and R6 are independently:
(a) hydrogen
(b) (C1–C6)-alkyl, or
(c) alkali-metal or alkali earth-metal
wherein the alkali-metal or alkali earth metal is sodium, potassium, lithium, cesium, rubidium, barium, calcium or magnesium;
comprising the steps of:
(1) charging a solution of carbon dioxide source having a pH range of about 6.0 to about 12.0 into a reaction vessel;
(2) adding an effective amount of a mole ratio of a base and an active ingredient into the reaction vessel containing the solution of carbon dioxide source to maintain pH at about 6.0 to about 9.0 and a temperature range of about –3° C. to about 15° C.; [and]
(3) lyophilizing the solution of Step (2) to yield the final formulation product of a compound of formula Ia with less than about 10% of moisture content.
Merck asserted claims 21-34 asserted of the '150 patent and U.S. Patent No. 5,952,323; the District Court found the asserted claims of the latter patent not invalid and infringed. The District Court found the asserted claims of the '150 patent would also be infringed but were invalid for obviousness over the '323 patent in combination with PCT publication WO 98/18800. According to the District Court, although none of the three recited steps of the claimed process were disclosed in the prior art, the "recipe" for the final stabilized ertapenem formulation was disclosed, and the three recited steps were conventional manufacturing steps that were the product of routine experimentation. Specific conditions known in the prior art were:
• That formation of a carbon dioxide adduct is pH dependent and occurs between pH 6.0 and pH 9.0;
• That the pH could be adjusted using sodium hydroxide; and
• The adduct could be produced by lyophilization.
Although the temperature range limitation was not expressly taught by the cited art, the District Court found that it was known in the art that low temperatures minimized degradation, and as a consequence the skilled worker would want to keep the temperature as low as possible without freezing.
With regard to the objective indicia of non-obvious, the District Court held that although there was evidence of commercial success and copying, the evidence was not strong enough to overcome the "strong prima facie case of obviousness" established by the defendant. With regard to commercial success, the strength of the evidence was diminished because Merck was the exclusive licensee of the patent on ertapenem itself -- US. Patent No. 5,478,820. And with regard to copying, Hospira adduced evidence that it attempted five different processes to produce stabilized ertapenem before choosing the method that, if valid, would have infringed the '150 patent.
The Federal Circuit affirmed in an opinion by Judge Lourie joined by Judge Hughes, Judge Newman dissenting, setting forth a pithy statement of what constitutes obviousness:
Obviousness is a question of law, based on underlying factual findings, including what a reference teaches, whether a person of ordinary skill in the art would have been motivated to combine references, and any relevant objective indicia of nonobviousness. Apple Inc. v. Samsung Elecs. Co., 839 F.3d 1034, 1047–48, 1051 (Fed. Cir. 2016) (en banc).
The District Court, according to Merck, based its decision on the grounds that the skilled worker would have relied on "knowledge, creativity, and common sense" to arrive at the claimed invention, which considerations, according to Merck, should be limited to the question of whether there was a motivation to combine prior art references and not the ultimate question of whether an invention is obvious. As Merck explained the prior art, It was focused on the dimerization basis for instability, not hydrolysis, and "pH values favorable for reducing dimerization result in increased hydrolysis, and vice versa," thus precluding the required reasonable expectation of success.
Hospira argued that the skilled worker would have performed adduct formation as recited in the claims, to minimize subjecting the unstable ertapenem compound to conditions that would cause it to degrade. The panel majority found no clear error in the District Court's finding that the prior art taught: 1) minimizing dimerization by forming the carbon dioxide adduct of ertapenem at pH 6.0–9.0; 2) that sodium hydroxide could be used to adjust the pH; and 3) that the final adduct was to be obtained using "standard lyophilization techniques" (the claim recites no particular lyophilization methods or conditions).
The fact that the prior art was silent as to methods for minimizing hydrolysis was not persuasive because the solution recited in the claims "constitutes nothing more than conventional manufacturing steps that implement principles disclosed in the prior art."
It was undisputed that the combination of the '323 patent and the '800 PCT publication taught exposing ertapenem to carbon dioxide in a solution having pH between 6.0 and 9.0 followed by lyophilization, and that minimizing the temperature would have been a routine precaution. The opinion states the majority's basis for finding deficient Merck's arguments against its claims being obvious:
Merck's purported solution for minimizing both hydrolysis and dimerization was to create the carbon dioxide solution first, at the pH range disclosed in the prior art; then simultaneously add the ertapenem and a base to the solution, in order to maintain the pH range taught by the prior art; maintain a low temperature during the process; and lyophilize the final product to contain less than 10% moisture content. The only elements of that process that were not expressly disclosed in the prior art are emphasized in italics above—namely, the order of the steps, the simultaneous addition of base, the specific temperature range, and a final moisture content of less than 10%. But, as the court found, those are all experimental details that one of ordinary skill would have utilized via routine experimentation, armed with the principles disclosed in the prior art.
The panel majority found no error with regard to the District Court's determination that the objective indicia did not rebut obviousness as found based on the asserted references. Nevertheless, the opinion states that just because there was another patent for which Merck was the exclusive licensee wasn't enough to properly discount evidence of commercial success. As the opinion explains, there are frequently a bundle of patents protecting a commercial product, due in part to USPTO decisions during prosecution (restriction requirements, for example), as well as improvements in a product or process). Thus, the existence of "multiple patents do not necessarily detract from evidence of commercial success of a product or process, which speaks to the merits of the invention, not to how many patents are owned by a patentee." But here, the panel majority did not understand there to be clear error in the District Court's determination that the evidence of commercial success was not sufficient to overcome Hospira's prima facie case of obviousness.
On the question of evidence of copying, neither the District Court nor the panel majority was persuaded by Hospira's contention that copying was not relevant in the ANDA litigation context (because the FDA requires a generic drug manufacturer to copy an approved drug). In this case, the panel majority noted that the FDA does not "require the generic manufacturer to copy the NDA holder's process of manufacturing the drug." But the panel agreed with the District Court that Merck's evidence of copying did not overcome Hospira's prima facie case of obviousness.
Judge Newman dissented, saying that "[i]t is time to remedy our inconsistent treatment of the procedures and burdens in applying the evidentiary factors of obviousness" by returning to the "statutory rigor" imposed by the Supreme Court in Graham v. John Deere Co., 383 U.S. 1 (1966). In her view, the Court mandated in Graham that all of the Graham factors ("(1) the scope and content of the prior art; (2) the differences between the claimed invention and the prior art; (3) the level of ordinary skill in the field of the invention; and (4) objective ("secondary") considerations such as commercial success, failure of others, and long-felt need") are to be considered, particularly with regard to their effect on the analysis of the other factors. Judge Newman appreciates that the Federal Circuit has "properly" applied the Graham factors in certain cases (including Apple Inc. v. Samsung Electronics Co., 839 F.3d 1034, 1048 (Fed. Cir. 2016) (en banc); In re Cyclobenzaprine Hydrochloride, 676 F.3d 1063, 1077 (Fed. Cir. 2012); and Leo Pharmaceutical Products, Ltd. v. Rea, 726 F.3d 1346, 1357–58 (Fed. Cir. 2013), and in her view this approach was validated by the Supreme Court in KSR International Co. v. Teleflex Inc., 550 U.S. 398, 399 (2007). But she recognizes that some Federal Circuit cases have adopted the position used by the District Court and the panel majority here, that the objective indicia are to be used to rebut a prima facie obviousness determination based on consideration of the other three. Doing so shifted "the placement and [] burden of proof" improperly according to Judge Newman's dissent.
The proper role of the objective indicia, according to Judge Newman, was to be used as "independent evidence of non-obviousness," citing Ortho-McNeil Pharm., Inc. v. Mylan Labs., Inc., 520 F.3d 1358, 1365 (Fed. Cir. 2008), and reiterates the Federal Circuit's earlier observation that the "objective indicia 'may often be the most probative and cogent evidence in the record," and are 'to be considered as part of all the evidence, not just when the decision-maker remains in doubt after reviewing the art,'" citing Stratoflex, Inc. v. Aeroquip Corp., 713 F.2d 1530, 1538–39 (Fed. Cir. 1983). Judge Newman cites several cases to illustrate her point that the Federal Circuit has strayed from this proper use of the objective indicia, including Cubist Pharmaceuticals, Inc. v. Hospira, Inc., 805 F.3d 1112, 1130 (Fed. Cir. 2015); Novo Nordisk A/S v. Caraco Pharmaceutical Laboratories, Ltd., 719 F.3d 1346, 1353 (Fed. Cir. 2013); Wm. Wrigley Jr. Co. v. Cadbury Adams USA LLC, 683 F.3d 1356, 1364 (Fed. Cir. 2012); Otsuka Pharmaceutical Co. v. Sandoz, Inc., 678 F.3d 1280, 1296 (Fed. Cir. 2012); Tokai Corp. v. Easton Enterprises, Inc., 632 F.3d 1358, 1370 (Fed. Cir. 2011); Wyers v. Master Lock Co., 616 F.3d 1231, 1246 (Fed. Cir. 2010); Muniauction, Inc. v. Thomson Corp., 532 F.3d 1318, 1327 (Fed. Cir. 2008); Aventis Pharma Deutschland GmbH v. Lupin, Ltd., 499 F.3d 1293, 1302 (Fed. Cir. 2007); and Ormco Corp. v. Align Tech., Inc., 463 F.3d 1299, 1311 (Fed. Cir. 2006).
While consistent with earlier application of the Graham factors, Judge Newman somewhat ironically neglected to mention the Federal Circuit's decision (from which she also dissented) in Pharmastem Therapeutics, Inc. v. Viacell, Inc. (Fed. Cir. 2007), a case decided close upon the Supreme Court's KSR decision. In that case, the panel majority (Judges Proust and Bryson) ignored exceptionally strong evidence on objective reasons for finding the claimed invention nonobvious, similarly relying on "strong prima facie" evidence that the prior art (which did not disclose the claimed methods or hematopoietic stem cell compositions) rendered the claims obvious.
This practice needs reform, because in Judge Newman's view:
It is time to restore conformity to precedent, in the interest of stability of practice and procedure, and predictability and fairness of result. I would reestablish the proper analytic criteria under the four Graham factors, and would remand to the district court to apply the correct law.
Merck Sharp & Dohme Corp. v. Hospira, Inc. (Fed. Cir. 2017)
Panel: Circuit Judges Newman, Lourie, and Hughes
Opinion by Circuit Judge Lourie; dissenting opinion by Circuit Judge Newman