
On Friday, December 7th, the Federal Circuit handed down two opinions concerning the proper application of the judicially created doctrine of obviousness-type double patenting (OTDP). The first, Novartis AG v. Ezra Ventures LLC (Fed. Cir. 2018), set forth the narrow, albeit important, holding that a terminal disclaimer, or loss of term for a later-expiring patent based on an earlier-expiring patent in relation to which the later-expiring patent recited patentably indistinct claims, did not include extension of term pursuant to the provisions of 35 U.S.C § 156. The Court held that, insofar as patent term extension under § 156 was a statutory grant, and loss of term under OTDP was a judicially created doctrine, the statutory grant was not trumped by OTDP.
The second case decided by the Federal Circuit that day, Novartis Pharmaceuticals Corp. v. Breckenridge Pharmaceutical Inc., provided the Court with the opportunity to decide whether the operation of the OTDP doctrine was the same for two patents that were granted under U.S. patent law after the term of a U.S. patent was changed from 17 years from the grant date to 20 years from the earliest claimed priority date under the Uruguay Round Agreements Act of 1994 (URAA), compared with circumstances where one patent is subject to pre-URAA term and the other to the post-URAA term.
The case arose in ANDA litigation concerning Novartis's Zortress® and Afinitor® drugs (active ingredient: everolimus), used to treat cancer and prevent rejection in kidney and liver transplantations. Before the District Court, Breckenridge Pharmaceutical challenged the validity of Orange Book-listed patent in suit (to the compound), U.S. Patent No. 5,665,772, based on obviousness-type double patenting. The invalidating reference, Novartis's U.S. Patent No. 6,440,990 (directed to methods of treatment using pharmaceutical compositions of the claimed compound), was filed after but issued before the '772 patent (a fact pattern akin to that before the Court in Gilead Sciences Inc. v. Natco Pharma Ltd.); the relationship between the two Novartis patents is illustrated below:
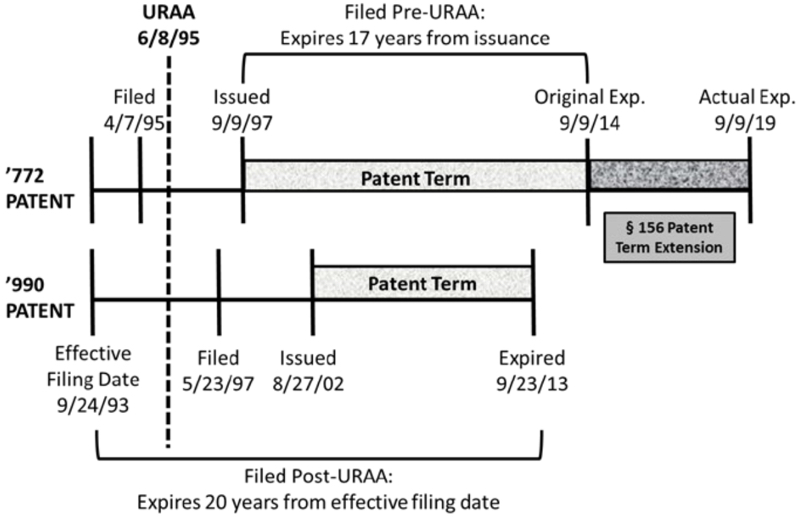
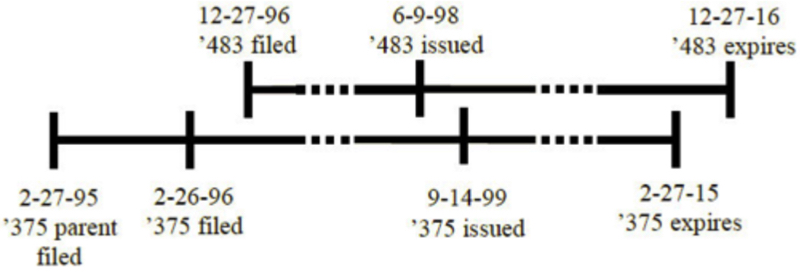
This pattern is in contrast to the relationship between the two patents in the Gilead decision, a difference important to the Federal Circuit's decision here:
Despite these differences, the District Court, relying on the rubric from Gilead that "a later-filed but earlier-expiring patent can serve as a double patenting reference for an earlier-filed but later-expiring patent," invalidated the '772 patent claims under OTDP over the '990 patent claims. As explained in the opinion, the District Court also relied on decisions by district courts following Gilead, including Janssen Biotech Inc. v. Celltrion Healthcare Co., 210 F. Supp. 3d 278 (D. Mass. 2016); MLC Intellectual Property, LLC v. Micron Technology, Inc., 2016 WL 4192009 (N.D. Cal. Aug. 9, 2016); and DDB Technologies, LLC v. Fox Sports Interactive Media, LLC, 2014 WL 12167628 (W.D. Tex. May 15, 2014), in support of its decision. The District Court rejected four arguments asserted by Novartis attempting to distinguish Gilead. First, that grant of the '990 patent did not affect the expiration date of the '772 patent; the Court held that "it was Novartis's choice to file the '990 patent, and the harm to the public lies in the inability to practice the invention claimed in the '990 patent once it expired." Second, that Novartis had not engaged in any gamesmanship which was the basis for the Gilead court's concerns; the Court held that gamesmanship was not required to violate proscription against OTDP under Gilead (or AbbVie, Inc. v. Mathilda & Terence Kennedy Institute of Rheumatology Trust, 764 F.3d 1366 (Fed. Cir. 2014)). Third, that allowing the earlier-expiring, post-URAA patent to be an OTDP reference against a later-expiring, pre-URAA patent would impermissibly shorten the statutorily mandated 17-year term of the pre-URAA patent; the Court found that Novartis had courted that risk by filing the earlier-expiring patent having patentably indistinct claims. Finally, that the patent term extension Novartis had obtained for the '772 patent "immunized" the patent from a double patenting challenge; the Court found no precedent for this contention and noted that the question was not at issue. The parties having stipulated to infringement and that the '772 patent claims would be invalid for OTDP if the '990 was a proper OTDP reference, the District Court entered judgment that the '772 patent was invalid and this appeal followed.
The Federal Circuit reversed, in an opinion by Judge Chen joined by Chief Judge Prost and Judge Wallach. The panel held that their decision in Gilead did not control in this instance, because the difference between how the patents were related to one another with respect to whether their expiration dates made a difference in how the doctrine of OTDP should be applied. According to the opinion:
[T]he correct framework here is to apply the traditional obviousness-type double patenting practices extant in the pre-URAA era to the pre-URAA '772 patent and look to the '772 patent's issuance date as the reference point for obviousness-type double patenting.
Under their analysis, "because a change in patent term law should not truncate the term statutorily assigned to the pre-URAA '772 patent," the '990 patent "cannot properly be used as an OTDP reference."
One aspect of this decision useful for the bar is the careful explication of how courts have "applied the principles of obviousness-type double patenting for over a century to restrict a patent owner's patents on an invention and obvious variants to one 17-year patent term," particularly with regard to the issuance dates of earlier- and later-expiring patents. Gilead, according to the opinion, represents a realization by the Court:
[T]hat the change in patent term law under the URAA altered the analytical inquiry for double patenting; issuance dates of post-URAA patents did not serve as reliable stand-ins for the expiration date of the patent as is true for pre-URAA patents, and the proper reference point for an obviousness-type double patenting inquiry is the expiration date of the patent in question.
The circumstances before the Court here are not the same and Gilead does not control, according to the opinion ("Gilead addressed a question that is not applicable here"). And the opinion characterizes the Court's Gilead opinion as being dependent upon the changes that arose post-URAA, because patent issue dates no longer "served as a reliable stand-in for the date that really mattered -- patent expiration." This is because, as in the Gilead case, "a patent that issues first does not [necessarily] expire first." The relationship of the earlier- and later-expiring patents before the Court here also does not have the potential negative consequences the Court cautioned against in Gilead, such as the opportunity for patentees to engage in gamesmanship regarding patent application and issue dates, and the potential that a difference of even one day in issue date could change the relationship in an OTDP-determining way.
The panel also concluded that the Court's AbbVie decision did not control the outcome. The relationship of the earlier- (U.S. Patent No. 7,846,422) and later- (U.S. Patent No. 6,270,766) expiring patents (both having post-URAA filing dates) in that case was as follows:
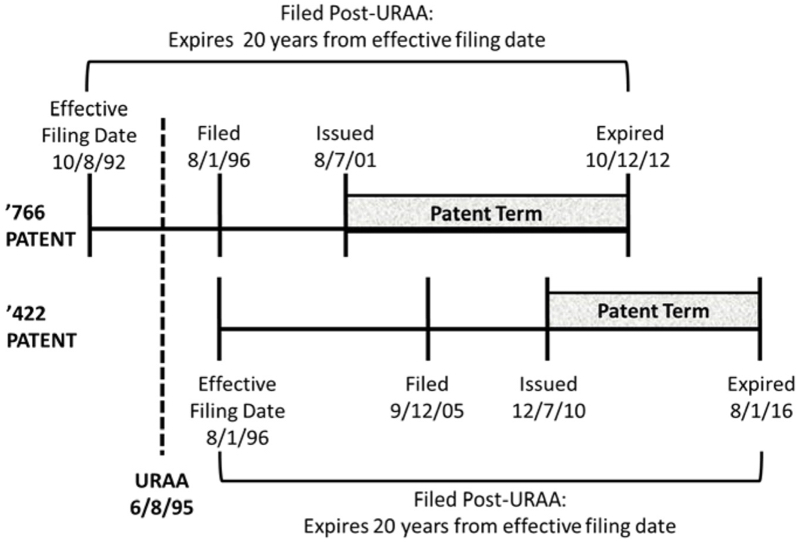
The AbbVie situation is "a prime example of the post-URAA scenario [the Court] contemplated in Gilead," according to the opinion (noting that, as in Gilead, the effective filing date (EFD) of the '422 patent represents applicant choice, because it did not have the same EFD of the '766 patent). That factor was not at play regarding the Novartis patents and thus AbbVie does not control the outcome here.
The Federal Circuit also found support (and solace) for its decision in the choice Congress made in the URAA transition statute, which gave applicants a patent term that was "the greater of" 17 years from issue date or 20 years from the effective filing date. Thus evinced a choice by Congress for patentees "to enjoy the maximum possible term available for their resulting patents under either patent term regime." Breckenridge's position, that OTDP could be used to "truncate any portion of the statutorily assigned term of a pre-URAA patent that extends beyond the term of a post-URAA patent would be inconsistent" with this Congressional choice. The panel also believes the outcome here is consistent with the "core principle underlying the double patenting doctrine: giving one invention and nonobvious variants of that invention the same patent term." Stating its rationale succinctly, the Court said:
The key purpose of obviousness-type double patenting is to prevent a patent owner from extending the exclusivity rights over his invention beyond a full patent term. We saw this impermissible practice in Gilead and in AbbVie, where the patent owners claimed different effective filing dates for different patents to extend the life of patent exclusivity. Gilead[.] Here, critically, Novartis did not seek to extend its patent rights over its everolimus invention beyond one patent term, in this case, 17 years from issuance of the '772 patent. Had the law not changed, regardless of whether Novartis obtained the '990 patent, the '772 patent would have expired on September 9, 2014 (September 9, 2019 with the patent term extension). The fact that the law for the term of a patent changed, resulting in the later-issued '990 patent having an earlier expiration date than it would have pre-URAA should not affect the '772 patent's statutorily-granted 17-year patent term. Rather than Novartis receiving a windfall with a 17-year term for its '772 patent, its '990 patent's term was truncated by the intervening change in law. To find that obviousness-type double patenting applies here because a post-URAA patent expires earlier would abrogate Novartis's right to enjoy one full patent term on its invention (citations omitted).
Novartis Pharmaceuticals Corp. v. Breckenridge Pharmaceutical Inc. (Fed. Cir. 2018)
Panel: Chief Judge Prost and Circuit Judges Wallach and Chen
Opinion by Circuit Judge Chen