Dennis Crouch, our colleague at Patently-O, tweeted last week that there have been 148 U.S. patents granted having disclosure related to (COVID-19 or SARS-CoV-2); see Search of U.S Patent and Trademark Office Patent Full-Text and Image Database and
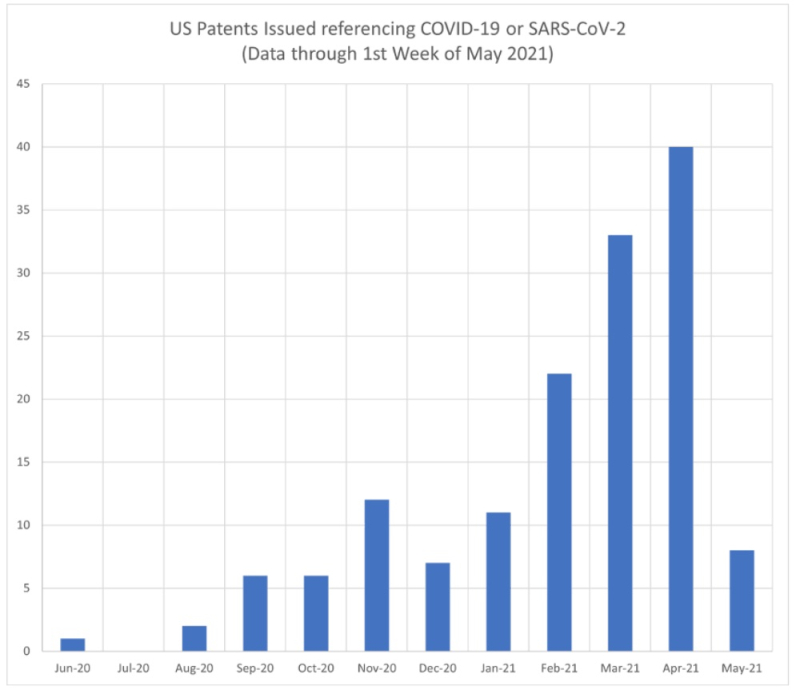
With regard to issues involving the proposed WTO IP waiver (see "If the Devil of the WTO IP Waiver Is in the Details, What Are the Details?"), it may be instructive to inspect the relatively few (4) of these patents directed to COVID-19 specific vaccines (another 4 were directed to therapeutic antibodies). These are set forth in this table:
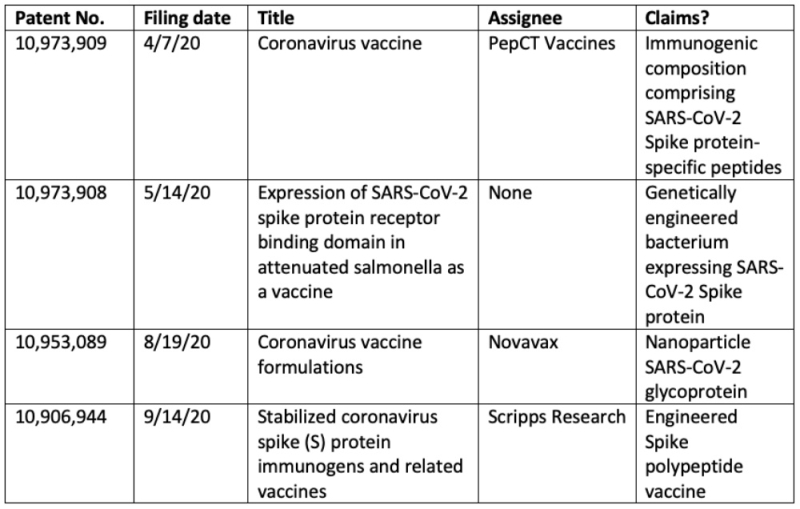
Of these, the only assignee associated with an actual, in-development SARS-CoV-2 vaccine is Novavax; however, the company has delayed its application for Emergency Use Authorization (EUA) from this month to sometime in the third quarter of this year (although maintaining that there is nothing troubling about the delay).
In addition to these granted patents, there are 420 published applications that are found on the USPTO website when queried with (COVID-19 or SARS-CoV-2); the same pattern persists here, with only a small fraction being directed to vaccines or immunization methods:
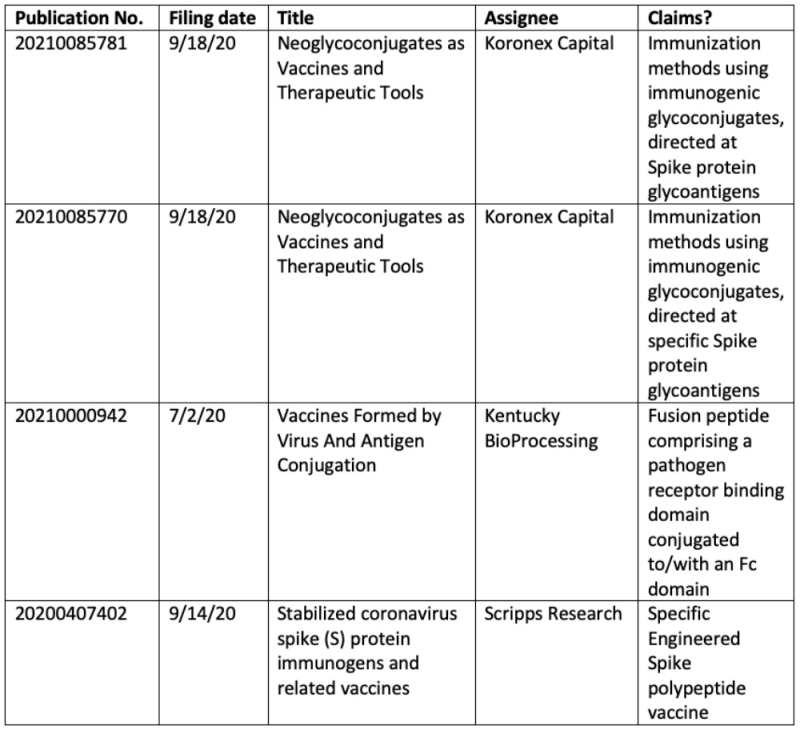
As with granted patents, the remainder of these pending applications are directed to detection methods, personal protective equipment, disease monitoring, and disinfectant preparations, as well as various pharmaceuticals and methods of treatment (not all of which are COVID-19 specific).
This raises the question of whether the uproar over the proposed WTO waiver is "much ado about nothing" in view of the small number of granted patents and pending applications directed to COVID-19 vaccines. A proper understanding of what is at stake involves considerations much more broadly shaped than these small number of patent properties. For example, there are many other patented technologies involved in making any of the vaccines now available:
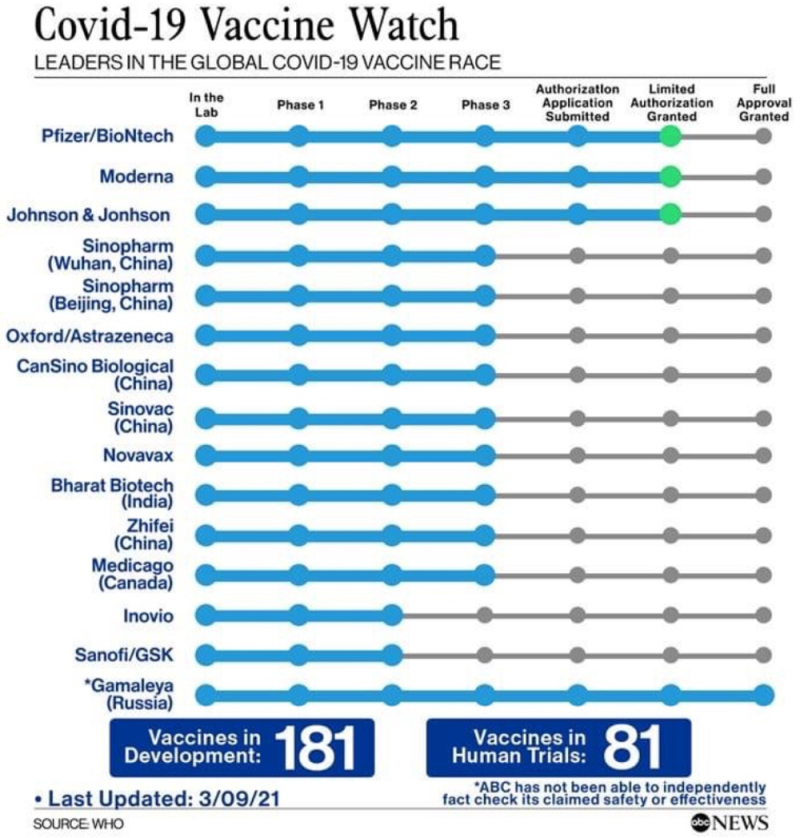
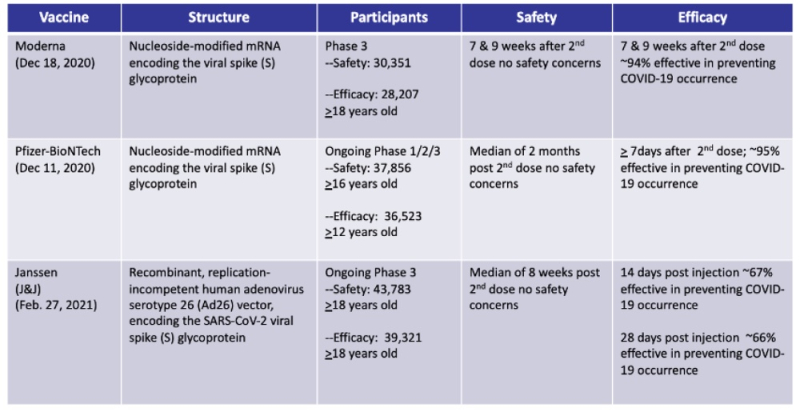
U.S. Food and Drug Administration EUA Information
In addition, these vaccines are also produced using trade secrets that are undoubtedly not disclosed in these patents (see "Suspending IP Protection: A Bad Idea (That Won't Achieve Its Desired Goals").
Most importantly, the cure is worse than the disease; as has been enunciated heretofore, the proposed IP waiver will do little if anything to solve the accessibility problem (see "Latest COVID Conundrum: Accessibility of Vaccines (When They Are Available)") but will almost certainly damage the very innovation system whose existence permitted these vaccines and others now in development to be produced on an accelerated timescale (see "The Road to Hell Is Paved with What Everybody Knows").
The Biden administration has had the benefit of learned, reasoned calls (see "Pfizer CEO Pens Open Letter on COVID-19 Vaccine IP Waiver"; "BIO & IPO Issue Statements on Biden Administration's Support for Proposed WTO Waiver"; "Sen. Tillis Asks Biden Administration to Oppose WTO Waiver Proposal"; "IP Organizations Support Continued Opposition to Waiver Proposal") not to scramble willy-nilly down the rabbit hole enticingly proposed by those who may have good hearts but other with less than clear motives. One of the nascent hallmarks of this administration (as compared with the last one) is an ability to recognize a blunder and correct it; after all, that is what political professionals who aspire to be statesmen are supposed to be able to do. The negotiation period for any proposed WTO IP waiver gives this administration the opportunity to achieve the desired goal (global COVID-19 vaccination) without harming the global innovation system. It is an opportunity the President and his administration should take.