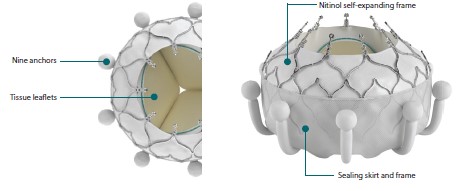
The U.S. Food and Drug Administration (“FDA”) recently approved the Edwards EVOQUE® Tricuspid Valve Replacement System (the “EVOQUE® system”) for use in treating Tricuspid Regurgitation (“TR”). The EVOQUE® system is designed to replace the native tricuspid valve in patients suffering from severe TR without the need for conventional open-heart surgery, according to the system’s Instructions For Use. As shown in the following illustrations from page 5 of the EVOQUE® system’s Patient Guide, the EVOQUE® valve is made of a nitinol self-expanding frame, an intra-annular sealing skirt and anchors, and tissue leaflets made from bovine pericardial tissue.
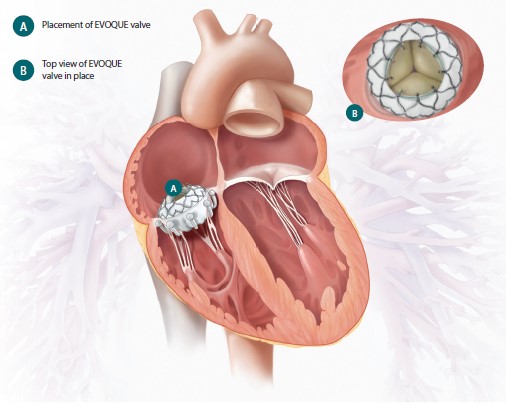
The EVOQUE® system is placed using a delivery system via femoral venous access to the tricuspid valve. The following image from page 6 of the EVOQUE® system Patient Guide illustrates the placement of the EVOQUE® valve within a patient’s heart.
In response to the FDA’s approval of the EVOQUE® system, Edwards Lifesciences transcatheter mitral and tricuspid therapies corporate vice president, Daveen Chopra, stated in a press release:
We are grateful for the strong collaboration with clinicians all over the world who contributed to the EVOQUE® system now being available through FDA’s Breakthrough Pathway to provide a treatment option to the many patients in the US suffering from tricuspid valve disease.
The EVOQUE® system was studied in a clinical trial entitled “TRISCEND II trial,” according to the FDA Summary of Safety and Effectiveness Data. As shown in the Construct of the TRISCEND II Trial Data Analysis Plan Venn diagram below from page 14 of the FDA Summary, the trial was designed to have a phased primary analysis plan with a Full Cohort of 400 patients including a Breakthrough Pathway Cohort subset of 150 patients.
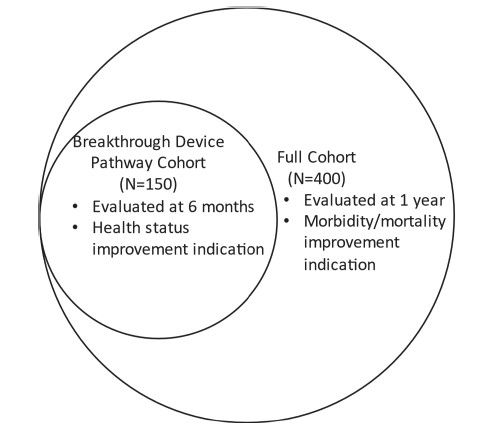
In the Breakthrough Pathway Cohort, the primary effectiveness endpoint #1 showed that the proportions of patients with TR reduction to moderate or less at six months were 98.8% in the device group, compared to 21.6% in the control group (p < 0.001). In the Full Cohort, the 1-year results available at the time of the FDA Summary showed favorable trends in the device group compared to the control group in various primary endpoint components. According to page 46 of the FDA Summary, the data from the PMA application is sufficient to “support the reasonable assurance of safety and effectiveness of the EVOQUE® system for the improvement of health status in patients with symptomatic severe TR who are refractory to OMT.”
According to Medi-Tech Insights, the tricuspid valve device market size is growing as the healthcare community recognizes the significance of tricuspid valve diseases and complications. Specifically, Medi-Tech asserts that the Global Tricuspid Valve Repair Market is expected to reach a US$2.5 billion market by 2028, as North America has emerged as the predominant global market for tricuspid valve repair due to the U.S. regulatory environments, technological advancements, and the growing needs among the geriatric population.