Orthobond received on April 5th de novo approval for the company’s spinal fusion device with quaternary ammonium compound coating.
Orthobond’s antibacterial treatment, Ostaguard™, covalently bonds its antimicrobial, polycationic molecules to the surface of an implant before packaging and sterilization. Orthobond claims that its patented approach requires dunking a medical implant such as a screw or replacement part in a chemical bath and then heating the device.
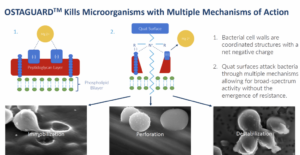
Orthobond’s process bonds a phosphonic acid layer only 4 or 5 nanometers thick to the oxide on the surface of the implant. Then an approximately four-hour polymerization process attaches quaternary ammonium molecules with densely packed, positively charged nitrogen at the end of the chain. The resulting layer of positively charged, quaternary ammonium molecules on an implant’s surface measures only one-millionth of an inch and immobilizes, perforates and destabilizes microbes.
(Image from www.medicaldesignandoutsourcing.com/orthobond-ostaguard-antimicrobial-coating-implant-device-chemistry-applications/)
Orthobond CEO David Nichols described the effect of the positively charged layer of quaternary ammonium molecules:
It’s like flypaper because bacteria is negatively charged and our surface is positively charged. It actually draws them to our surface, puts little pin pricks in the bacteria and starts the death process. As they try to move around, it breaks them apart and kills them.
Studies have shown that between 70-100% of explanted hardware from failed joint implants have some level of contamination from different sources. Orthobond claims its Ostaguard™ technology is highly effective in killing 12 microbes representing a majority of device-related infections. Ostaguard does not use antibiotics which, according to David Nichols, reduces the risk of creating drug-resistant bacteria and does not present the same toxicity issues of antimicrobial materials such as silver palladium, and copper.
The FDA assigned a new product classification, defining the category as “a rigid metallic implant device or system comprised of single or multiple components intended to facilitate fusion in skeletally mature patients. The device includes a quaternary ammonium compound coating that is covalently bonded to the device. Where applied, the coating is intended to reduce microbial contamination on the surface of the device prior to implantation. The device does not contain antimicrobial agents that act within or on the body and this device type does not include combination products.” The first application of the Ostaguard™ technology will be on SeaSpine Mariner pedicle screws. The FDA classified the SeaSpine Mariner pedicle screws as a Class II device and established special controls, which Orthobond declined to divulge.