
In January, the Federal Trade Commission issued a report on the terms of settlement agreements between branded and generic drug companies in ANDA litigation under the Hatch-Waxman Act, according to the provisions of the Medicare Prescription Drug, Improvement, and Modernization Act of 2003. While the Commission has been issuing these Reports since the MMA was enacted, this year's report is significant, because it is the first Report issued by the FTC after the Supreme Court's decision in FTC v. Actavis, which held that under some circumstances such settlement agreements containing so-called "reverse payments" could violate the antitrust laws.
According to the report (which can be accessed here, there were 160 settlement agreements between branded pharmaceutical companies and generic competitors filed in Fiscal Year 2014. This number, according to the Report, is "substantially the same" as the number of settlement agreements in recent years. Specifically, the Report notes that by comparison there were 156 agreements filed in FY 2011, 145 in FY 2012, and 140 in FY 2013. As set forth in the Report, of the 160 agreements reported to the Commission in FY 2014:
• 21 contained "potential" reverse payment provisions, involving 20 different drugs with sales totaling $6.2 billion;
• 10 of these 21 involved compensation "solely in the form of cash," in amounts ranging from $35,000 - $5 million;
• 6 of these 21 involved a "side" business deal between the parties;
• 5 of the 21 included a promise from the branded pharmaceutical not to market an authorized generic;
• 8 of the agreements contained ambiguous provisions, for example, related to "a declining royalty structure" that could act like an agreement not to market an authorized generic;
• Of the rest, (111 of 160) the agreements restricted a generic company's ability to market their products but do not contain either explicit or "possible" compensation from the branded to the generic; and finally
• 20 of the settlements contained no provisions limiting generic entry.
What has changed is the number of these agreements containing reverse payment provisions, which "decreased significantly" (by about 50%). The trend is clear: there were 31 agreements containing reverse payment provisions in FY 2010, 28 in FY 2011, and 29 in FY 2013. The trend is most pronounced for "first filers," those generic manufacturers who are first to file an ANDA application. For first filers there were 18 agreements in FY 2011, 23 in FY 2012, and 13 in FY 2013, compared with 11 in FY 2014. There has also been a reduction in the number of agreements involving first filers containing agreements by the branded drug maker not to market an "authorized generic" in competition with the generic entrant. These provisions are particularly important to first filers, who get 180 days of market exclusivity if they successfully challenge the branded company's patents; competition by a "generic" form of the drug made by the branded manufacturer can greatly reduce the short-term profits (and hence economic incentives) for the generic entrant. There were 15 agreements containing such provisions filed in FY 2010, 11 in FY 2011, 19 in FY 2012, and 4 in FY 2013, compared with 5 in FY 2014.
Overall, the Report reveals that 81-87% of ANDA litigation settlement agreements filed in FY 2014 did not contain any compensation from the branded to the generic company and/or restrictions on generic market entry. These results show that the outcome desired by the FTC in a decade of challenging these agreements that culminated in the Court's Actavis decision have borne the desired fruit. Whether this outcome facilitates generic entry and reduced drug costs for consumers is a conclusion the data do not yet support.
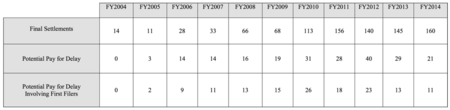
Exhibit 1 from Report (click to enlarge table)