The COVID-19 pandemic has spread throughout the globe, infecting more than 90 million people and causing almost two million deaths (see "Tracking coronavirus’ global spread"). SARS-CoV-2 infection is the cause of the COVID-19 pandemic; this virus is recognized as the latest viral infection in humans of zoonotic origins, in this case bats first arising in Wuhan, China. The infection is known to be introduced into the lungs, and infection due to the viral Spike protein binding to the angiotensin 1 converting enzyme 2 (ACE-2) receptor expressed in the lung.
Among the many consequences of SARS-CoV-2 infection are respiratory disease, as well as effects in the liver as well as neurological tissues. The capacity of SARS-CoV-2 to infect brain is supported by finding viral RNA and proteins in brain tissue on autopsy, but the frequency of neurological infection remains unknown. Regarding COVID-related disease in brain, there are a number of possible bases for the anecdotal reports of impaired function (including persistent headache and impaired consciousness and cognition), including inter alia reduced blood-borne oxygen due to impaired respiration. This is a particular risk in patients with mild disease symptoms, because humans sense lack of oxygen indirectly by increased levels of carbon dioxide in the blood and thus brain and other neurological tissues can be oxygen-deprived unknowingly. In addition, it has long been known that there can be psychological sequellae to severe, life-threatening, debilitating diseases and thus the effects of psychology rather than pathology is not easy to tweeze out of the clinical presentation of these effects.
This week, an international group of researchers* illuminated how SARS-CoV-2 can infect neurological tissue and provides a viral explanation of neurological COVID infection, in a paper entitled "Neuroinvasion of SARS-CoV-2 in human and mouse brain," in the Journal of Experimental Medicine. The experiments these researchers performed are illustrated in the paper's "graphical abstract":
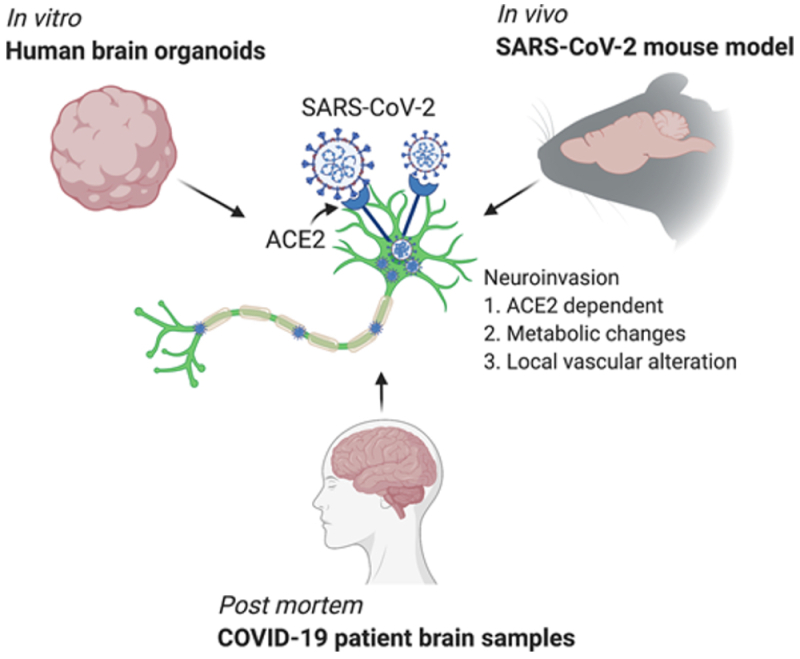
The first of these methods used human neural progenitor cells and human brain organoids produced therefrom, which the authors reference as having been used to study Zika virus infection (which had been associated with anencephaly in fetuses in utero). These experiments showed infection of 2-week old human neural progenitor cells, wherein peak viral titers were found 12 hours after infection and were associated with increased cell death. SARS-CoV-2 infection was found in 9-week old human brain organoids within 24 hours of infection and significant infection within 96 hours post-infection. "[T]he majority of the SARS-CoV-2–infected cells were localized within MAP2-positive cellular fields of mature neurons," according to the authors, but infection was also observed in "SOX2-positive neural stem cells with bipolar morphology and cells localized around the neural tube–like structures." Viral particles were observed by electron microscopy to be located intracellularly associated with endoplasmic reticulum, and were associated with "extensive neuronal cell death." The authors conclude from these organoid studies that "SARS-CoV-2 can infect cells of neural origin and suggested that infected cells can promote death of nearby cells."
Turning to molecular studies, the researchers reported investigation into whether some cells are more susceptible to virus-induced cell death than others, using single-cell RNA profiling. These experiments were performed using 60-day old organoids virus-infected or mock-infected, and 96,205 cells were assayed in 31 clusters comprising "neural progenitors/outer radial glia, intermediate progenitor/ interneurons, neurons, and cortical neurons." The researchers reported these experiments showed "widespread infectivity of SARS-CoV-2 in neurons, radial glia, and neuronal progenitor cells." When compared with the effects of Zika virus (ZIKV) infection on neural tissues, "SARS-CoV-2–infected brain organoid up-regulated pathways related to cell division, organelle fission, and metabolic processes, while ZIKV showed enrichment in type I IFN pathways." These results were consistent, according to these researchers, with reports that "SARS-CoV-2 induces a moderate IFN-stimulated gene response in other tissues" as well as "previous reports of specific virus replication being controlled by alternative pathways by neurons."
When comparing SARS-CoV-2 infected cells with adjacent uninfected cells, the infected cells showed "enrichment of genes corresponding to viral transcription, along with enrichment for metabolic processes including electron transport–coupled proton transport, cytochrome c to oxygen, and NADH to ubiquinone" whereas the uninfected cells showed "a mitochondrial catabolic state with the up-regulation of alcohol metabolism, cholesterol synthesis, and regulation of cell death." Consistent with the mechanism of infection in other tissues, experiments using ACE2 receptor-blocking antibodies showed that expression of ACE2 receptor was necessary for neural tissue infection, despite the low frequency of detecting ACE2 receptor mRNA expression in neural tissues. In addition, IgG antibodies immunologically specific for the viral Spike protein were found in cerebrospinal fluid from patients with virus-associated acute encephalopathy, which antibodies were capable of blocking virus infection in human brain organoids.
The authors also produced a mouse model of SARS-CoV-2 infection using transgenic mice that expressed human ACE2 in brain tissue. Intranasal administration of the virus produced infection in neural cells in the forebrain; in contrast there was "a relatively low density of infected cells [in] the dentate gyrus, the globus pallidus, and cortical layer 4." In perhaps the most disquieting result reported in this paper, mice induced to express human ACE2 receptor in either lung tissue or ventricular structures in brain by Adenovirus infection were shown in later SARS-CoV-2 infection to have "signs of lung pathology but no weight loss or death," but both weight loss and death caused by brain infection even at much lower (100-fold) administered virus dose, suggesting neurological infection has high "neuroreplicative potential and lethal consequences."
Finally, the paper reported results of three human autopsies of COVID-19 patients, all of which "had been admitted to the intensive care unit, . . . sedated and ventilated due to respiratory failure [within 3-18 days], and [wherein] their difficulty to be weaned from mechanical ventilation indicated the severity and highly pathogenic nature of the disease course." The patterns of virus-specific immunostaining were somewhat idiosyncratic, but death was associated with "a temporal sequence of continued ischemic events." Somewhat curiously, regions of SARS-CoV-2 infection were not associated with lymphocyte or leukocyte infiltration which indicates, according to these authors, that "although SARS-CoV-2 has neurotropic properties and can infect neurons in patients, it did not invoke an immune response typical of other neurotropic virus."
The paper concludes that "the brain is a site for high replicative potential for SARS-CoV-2," that "SARS-CoV-2 causes significant neuronal death in human brain organoids," that "ACE2 is expressed at the protein level and is functionally required for SARS-CoV-2 infection in human brain organoids," and that "there is robust antibody response against the virus within the CSF," which can cause "cascading downstream effects in causing and amplifying CNS inflammation." The mouse model studies reported "for the first time that SARS-CoV-2 neuroinvasion in mice can have significant remodeling of brain vasculature." Human autopsy results, according to this paper, showed that neural pathology in humans was a consequence of acute ischemic damage caused by the infection (although these researchers emphasized that the small sample size limited the conclusions that they could draw from these results). Despite these caveats, the paper concludes with these researchers asserting that:
Altogether, our study provides clear demonstration that neurons can become a target of SARS-CoV-2 infection, with devastating consequences of localized ischemia in the brain and cell death, highlighting SARS-CoV-2 neurotropism and guiding rational approaches to treatment of patients with neuronal disorders.
While gratifying to the extent that these researchers have explicated the ability of SARS-CoV-2 to infect neural tissues and produce neurological disease, the paper suggests that any hope that 2021 will be a better year than 2020 may be unfounded, or at least too much to hope for, as well as both a cautionary tale of the dangers of this infection and the importance of achieving effective vaccination quickly and universally.
* From the Departments of Immunobiology, Genetics, Pathology, Internal Medicine, Comparative Medicine, and Neuroscience, Yale Medical School; Hôpital Pitié-Salpêtrière and the Sorbonne, Paris.