Cancer, the "Emperor of All Maladies" as it has been termed, has been studied for millennia. President Nixon's "War on Cancer" resulted in slow but steady progress, aided by the biotechnology revolution, the development of monoclonal antibodies (see Herceptin®) and more recent developments in immunological interventions like CAR-T cells (see "Global CAR-T Cell Therapy Market (2021 to 2027) - Size, Forecasts, Trials and Trends -ResearchAndMarkets.com").
But the basis for many characteristics of cancer biology remain elusive, including what is classically termed the Warburg effect, i.e., the tendency of cancer cells to be dependent upon aerobic glycolysis instead of oxidative phosphorylation even in the presence of oxygen and thus not being an adaptation for low oxygen conditions as it is in normal cells. (Those with long memories will remember the saga of the eminent Efraim Racker being fooled by a graduate student who presented falsified evidence of a protein kinase cascade that regulated glycolysis.) This effect has been known since the 1920's (during the Golden Age of research into the biochemical basis of intermediary metabolism) when Otto Heinrich Warburg found that depriving tumor cells of oxygen and glucose resulted in cell death. But the change has remained phenomenology perhaps until now.
Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) which is an inherited form of kidney cancer is characterized by deficiency in fumarate hydratase (FH), which catalyzes the reaction:
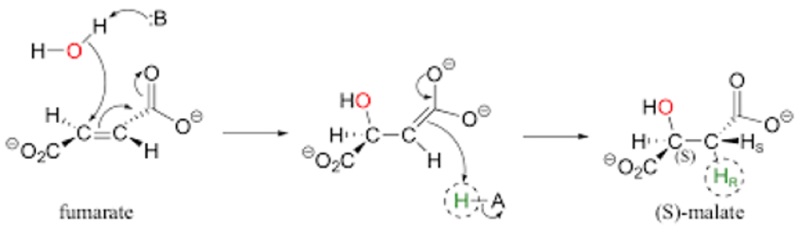
as part of the Krebs (TCA) cycle in the mitochondria. Until now there has been no understanding of why or whether this metabolic deficiency results in tumors that rapidly grow and metastasize. In a recent paper, a team of university and National Institutes' researchers* recently reported that the growth aggressive phenotype is associated with expression of the Warburg effect, in a paper entitled "Mitochondrial DNA alterations underlie an irreversible shift to aerobic glycolysis in fumarate hydratase-deficient renal cancer," in Science Signaling. These researchers show that the enzymatic deficiency is associated with an accumulation of fumarate in the cells, and this results in increased mutation rates in mitochondrial DNA as a consequence of fumarate accumulation. The authors draw the conclusion that these cancer cells are "rewired" to promote tumor progression and metastasis due to a loss of mitochondria and a "metabolic shift" to aerobic glycolysis.
FH is encoded by a nuclear-encoded gene but the enzyme is necessary for competent mitochondrially mediated metabolic function. The basis for high fumarate's deleterious effects on mitochondria is the "nonenzymatic formation of an S-(2-succinyl)-cysteine (2SC) adduct that inactivates multiple proteins involved in tumor biology and metabolism." The researchers found that there was a loss of respiratory-chain components encoded by mitochondrial DNA and with S-(2-succinyl)-cysteine (2SC) adduct-mediated inhibition of mitochondrial DNA replication. This in turn was caused by inactivation of the sole mitochondrial DNA polymerase as well as mtDNA maintenance and repair proteins, leading to mitochondrial depletion in these cells. The authors termed this mitochondrial depletion a "defining feature" of this tumor type and its aggressive growth and metastasis phenotype.
The researchers studied a cohort of 24 HLRCC patients aged 21-70 with the majority (75%) having advanced metastatic disease. Assessment of proteins in the respiratory chain showed disruption associated with morphological defects on the mitochondrial inner membrane (where these proteins are located in normal cells). As summarized by the authors, "[t]ogether, these data demonstrate an impairment in the assembly of respiratory CI, CIII, and CIV in HLRCC tumor mitochondria with a substantial loss of protein abundance of mitochondrially encoded subunits."
These results were consistent with the detected down-regulation of mitochondrial mRNA synthesis in these cells which was not seen in the expression of nuclear gene-encoded mRNAs members of Krebs cycle or respiratory chain proteins, as illustrated in this Figure:
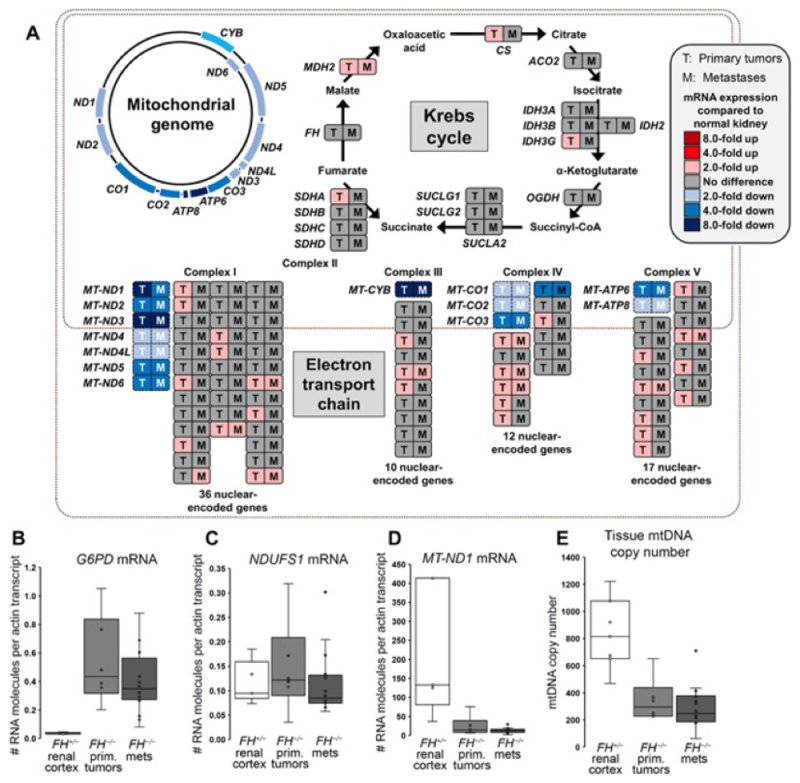
As stated in the paper "[o]ur transcriptome analyses indicated that the underlying cause of respiratory chain dysfunction in HLRCC FH−/− tumors was the selective loss of expression of mtDNA-encoded subunits" of the respiratory chain"; the authors were able to correlate this reduction in mtDNA gene expression with a reduction in the number of mitochondria per cell, from an average of 833 mtDNA molecules in normal renal cells to between 344 and 294 copies in primary and metastatic HLRCC tumors, respectively.
These changes were mirrored in HLRCC tumor-derived cell lines, albeit not entirely consistently. To evaluate these inconsistencies the researchers restored normal FH levels by introducing heterologous FH-encoding constructs that showed "[r]obust FH activity." Nevertheless, functional respiratory chain function was not established in these cells, consistent with the defect being associated with mitochondrial loss rather than merely FH disfunction: "[o]ur observations indicate that loss of FH activity had a lasting impact on mitochondrial function even after FH activity was restored and fumarate levels were reduced in the tumor-derived cells."
These results led the researchers to evaluate the status of mtDNA in these cell lines by DNA sequencing. These experiments showed multiple deactivating mutations in several mtDNA-encoded genes involved in respiration, including mutations not found in the Human Mitochondrial DNA database (HmtDB), which the authors assert suggested that "these mutations are not tolerated in normal human tissues." When similar experiments were performed in the HLRCC tumor samples, almost all (22) showed one or more somatic changes in mtDNA, each sample having on average 2.27 (range, 1-7) mutations in the mtDNA, wherein more than half of these mutations resided in protein-encoding mtDNA. Such patterns of mtDNA mutations were not found in other types of renal cell carcinoma not associated with FH deficiencies.
Finally, an analysis of the protein components of mtDNA, which encode proteins known to be involved in maintaining DNA integrity, were found by these researchers to be covalently modified by succinylation at cysteine (Cys) residues, resulting in their inactivation. These results were consistent with the loss of mitochondria population in these cells. These results were observed in both cells from HLRCC tumor cell lines and HLRCC patient samples.
These researchers summarized their results with this diagram:
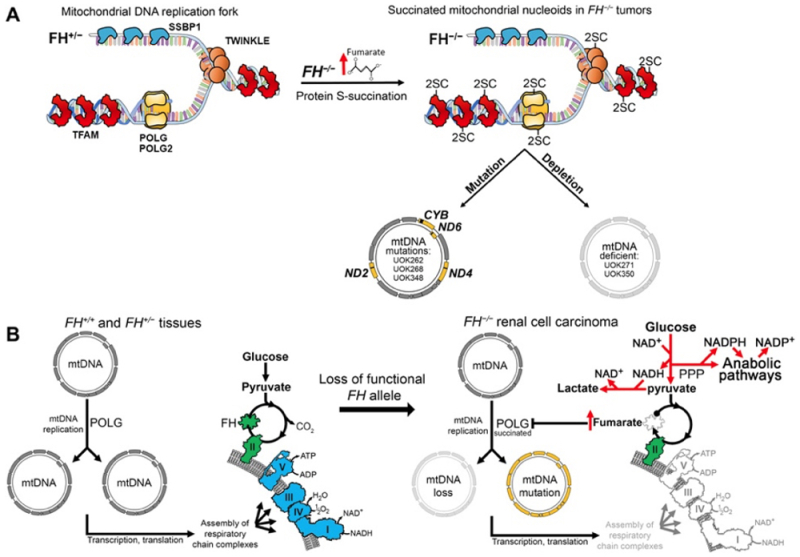
and by noting that FH deficiency resulted in inactivation of core enzymes involved in mtDNA integrity, repair, and replication and irreversible mitochondrial loss. Mitochondrial loss "obligates cells to compensate for energy loss by shifting to dependence on aerobic glycolysis as an energy source."
These changes were also associated with upregulation of enzymatic activity that promoted lipid synthesis, necessary for rapid tumor growth. As summarized by the authors:
[T]he metabolic compensations dictated by loss of mtDNA may result in features of the aggressive growth phenotype that characterizes HLRCC tumors, including increased fatty acid biosynthesis contributing to formation of new cell membranes, generation of the intermediates of the citric acid cycle that are used in anaplerotic reactions, and synthesis of other precursors necessary for biosynthesis of amino acids and nucleotides that are needed for rapid growth and invasiveness of this type of cancer.
And:
In summary, mitochondrial dysfunction forces metabolic remodeling in HLRCC tumors that favors anabolic pathways offering crucial bioenergetic and biosynthetic advantages for tumor growth and metastasis. Understanding that mtDNA mutations and deletions represent the fundamental cause for the shift to complete dependence on aerobic glycolysis in this aggressive form of inherited, genetically defined RCC will hopefully provide insights into the development of effective forms of therapy for patients with HLRCC and other related cancers.
* National Institutes of Health, National Cancer Institute, National Institute of Child Health and Human Development, Frederick National Laboratory for Cancer Research, and the University of Kentucky