 The U.S. Patent and Trademark Office spent the entire afternoon session of today's biotechnology/chemical/pharmaceutical (BCP) customer partnership meeting focusing on the guidance memorandum for determining the subject matter eligibility of claims under 35 U.S.C. § 101 that the Office issued on March 4 (see "USPTO Issues Guidance for Analyzing Subject Matter Eligibility of Claims Reciting Laws of Nature/Natural Principles, Natural Phenomena or Natural Products"). The Office spent nearly an hour discussing the Guidance, as well as the Training Materials that were released a few weeks after the Guidance, and then took questions from attendees and online viewers for more than an hour and a half.
The U.S. Patent and Trademark Office spent the entire afternoon session of today's biotechnology/chemical/pharmaceutical (BCP) customer partnership meeting focusing on the guidance memorandum for determining the subject matter eligibility of claims under 35 U.S.C. § 101 that the Office issued on March 4 (see "USPTO Issues Guidance for Analyzing Subject Matter Eligibility of Claims Reciting Laws of Nature/Natural Principles, Natural Phenomena or Natural Products"). The Office spent nearly an hour discussing the Guidance, as well as the Training Materials that were released a few weeks after the Guidance, and then took questions from attendees and online viewers for more than an hour and a half.
The Office's spokesperson for the initial presentation was June Cohen, a Legal Advisor with the USPTO's Office of Patent Legal Administration, who was joined during the question-and-answer portion of the session by Ali Salimi, also a Legal Advisor with the USPTO's Office of Patent Legal Administration, and Daniel Sullivan, a Supervisory Patent Examiner with the Art Unit 1611. Ms. Cohen began her presentation by noting that the Office would be hosting a public forum on the Guidance from 1:00 to 4:00 pm (ET) on May 9. Ms. Cohen also noted that the Office plans to collect public input via the forum and then "update or modify the Guidance as needed." The details regarding the forum have been posted on the Office's Myriad-Mayo Guidance webpage, which indicates that the forum is being held "to receive public feedback from organizations and individuals on the Guidance," and allow members of the public "to present their interpretation of the impact of Supreme Court precedent on the complex legal and technical issues involved in subject matter eligibility analyses during patent examination." The Office's Guidance webpage also indicates that "[p]articipants who believe that the Supreme Court decisions could be implemented in an alternative manner from the approach taken in the Guidance should use the forum to present their alternative approach and the legal rationale for the alternative." Participants have also been invited "to suggest additional examples for use by the Office to create a more complete picture of the impact of Supreme Court precedent on subject matter eligibility." The Office noted that registration will be required to attend the forum in person or via webcast (details for registering can be found on the Guidance webpage).
Following the announcement of the forum, Ms. Cohen dove into the Guidance, suggesting that the public had some "misunderstandings" regarding the Guidance that she hoped to clear up. She noted that the Office had received a lot of "public input" regarding the fireworks example (Example C of the Guidance), the scope of the second step in the Guidance's three-step analysis (i.e., Does the claim recite or involve one or more judicial exceptions?), and why the Office considered anything other than DNA when discussing natural products. With respect to the last question, Ms. Cohen explained that the Guidance was not only about Myriad or Mayo, but was about a whole host of cases, and that the Office took a comprehensive approach in the Guidance because the Supreme Court discussed these cases in both Myriad and Mayo. One of Ms. Cohen's slides provided a list of these cases:

and another slide showed, at least in part, how these cases are interrelated:
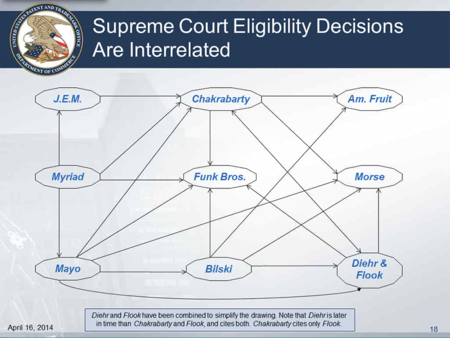
As a result, Ms. Cohen explained that there are many things that can be natural products, and that these things must then be analyzed under the third step of the process for determining subject matter eligibility:

Ms. Cohen also admitted that the Guidance is a "course correction" because the Office had received the message from the courts that some of the patents it had been issuing on DNA in the past were improperly issued (and presumably does not want to be admonished by the courts on other natural products in a piecemeal fashion).
With respect to the fireworks example, Ms. Cohen noted that the public response took the Office by surprise, and that the claim was adapted from an actual claim from the 1920's. She pointed out that there at least four types of gunpowder (simple mixture, corned gunpowder, glazed powder, and white powder), and that one of the four types (simple mixture) is "[a] mixture of three naturally occurring materials" wherein "[v]ibration causes separation back into its component parts," and therefore that "[s]uch a mixture is not markedly different because none of the components have been changed." Ms. Cohen indicated that the example was intended to demonstrate that because examiners must construe claims according to the broadest reasonable interpretation, examiners and applicants may be thinking about the meaning of gunpowder in different ways, as shown in one slide from her presentation:

Ms. Cohen also indicated that the Office was in the process of developing better examples than the fireworks example, which would not be as "confusing."
With respect to the three-step procedure described in the Guidance for analyzing claims for subject matter eligibility, Ms. Cohen noted that the heart of the analysis was not the second step, but rather the third step: Does the claim as a whole recite something significantly different than the judicial exception(s)? She also noted that the Office had received a lot of input about the meaning of the term "significantly different" in the third step, and one slide indicated that the "Guidance brings together the outcomes of both Myriad and Mayo in its expression of the 'significantly different' standard for eligibility." More importantly, because the Supreme Court has not created any bright line rules for determining subject matter eligibility, the Guidance does not involve the application of any bright line rule. Instead, examiners have been given a list of factors to consider, some weighing toward eligibility and some weighing against eligibility:

Ms. Cohen pointed out that the "markedly different" changes apply to structure because the Office found that functional change was usually tied to structural change. (During the question-and-answer portion of the session, someone pointed out that gunpowder was a good example of something for which the components were not structurally changed, but which when combined, had a significant change in function.) She also indicated that examiners had been warned not to jump to conclusions based on the inclusion of any particular claim terms (e.g., cDNA) because there are no magic words for establishing subject matter eligibility. Examples of some of these terms were provided in one slide:

Several questions during the question-and-answer portion were posed about claims directed to methods of treatment involving natural products (such as claim 3 in Example B of the Guidance, which recites a method of treating colon cancer using "amazonic acid"). With respect to the amazonic acid example, some questioners wanted to know why the Office decided to include dosages and course of administration, and whether this suggested that those limitations were necessary for subject matter eligibility. While Ms. Cohen explained that this was not the point of the example, she said that an analysis of a claim without those limitations should be conducted according to the Guidance and that factor (d) (Do more than describe the judicial exception(s) with the general instructions to apply/use it) could impact the analysis.
Another questioner drew an analogy between the fireworks example and vaccines, which also possess significant functional differences despite no change in the structure of their components (although the questioner was reluctant to admit that there were no structural changes in vaccines). The questioner noted that her company was already receiving § 101 rejections based on the Guidance on some of its vaccine claims. A different questioner suggested that the gunpowder example could be compared with the result in Funk Brothers, arguing that in the latter, natural products were combined with the components performing the same function, and in the former, natural products were combined with the combination performing a significantly different function. The questioner noted that many biotech combinations yield results that are superior as compared with the separate components.
Another questioner asked about the impact of the Guidance on antibodies, to which Ms. Cohen responded that if the antibody had been isolated (i.e., occurs in nature), then the antibody "is not looking very eligible," and that one way to make an antibody "look eligible" would be to engineer it. She also noted, however, that "many antibodies will be eligible absent evidence that [the antibody] is not naturally occurring." According to Ms. Cohen, examiners have been told not to speculate about whether something can be found in nature, and instead to produce evidence that something can be found in nature. In response to the hypothetical in which a claim recites something the applicant synthesized but that later turns out to be found in nature, Ms. Cohen explained that if the patent had not yet issued, the claim would be rejected for lack of subject matter eligibility, and if the patent had issued, then it would be up to the courts to invalidate the patent.
Towards the end of the session, Ms. Cohen acknowledged that the Office had not taken its task lightly when formulating the Guidance, and that the Office understands that the Guidance impacts "the livelihood of you, your clients, and their companies." Unfortunately, by putting the cart before the horse by only now seeking public input regarding the Guidance, the Office has not evinced such understanding. Maybe it has started.
Note: Ms. Cohen will be participating in a session at next week's Biotechnology Industry Organization (BIO) 2014 Spring Intellectual Property Counsels Committee (IPCC) Conference in Palm Springs, CA. The session, entitled "Facing the Future of Biotech Patenting: How to Satisfy the Supreme Court and Have Valuable Claims, Too," which is sponsored by McDonnell Boehnen Hulbert & Berghoff LLP, also features David Hoffman, Senior Corporate Counsel, IP & Transactions, Genomic Health; Laurie Hill, Vice President & Deputy General Counsel, MedImmune; and Patent Docs authors Kevin Noonan and Donald Zuhn.