The inherent, ineluctable unpredictability of biology can be the basis for biological patent claims being non-obvious (lacking the requisite "reasonable expectation of success"; see, e.g., OSI Pharmaceuticals v. Apotex) and for the greater quantum of disclosure necessary to satisfy the written description and enablement requirements of § 112 (see, e.g., Amgen v. Sanofi), despite complaints from the life sciences patent bar that these increased requirements are improper doctrinally and unfair. These two different characteristics can be frequently in tension for patenting in the life sciences, it being difficult to maintain on the one hand that there is insufficient expectation of success for a claim to be obvious and on the other hand that deficiencies in disclosure can be appropriately supplemented by the knowledge of one of ordinary skill.
This unpredictability was illustrated in a paper recently published in Nature Medicine, entitled "Common germline variants of the human APOE gene modulate melanoma progression and survival." These authors* showed (somewhat paradoxically) that one variant of the human APOE gene (APOE2) was associated with a propensity for tumor cells to metastasize, while a different variant (APOE4), which has been known for several years to be associated with development of Alzheimer's disease (see Strittmatter et al., 1993, Proc. Natl. Acad. Sci. USA 90: 1977-81), exhibited a metastasis-inhibiting effect (and APOE2 itself can have a protective effect on development of late-onset Alzheimer's; see Corder et al., 1994, Nat. Genet. 7: 180-84).
The experiments were performed in mice expressing human APOE4 or APOE2 by genetic replacement of the mouse analogs. Differences in melanoma tumor growth in these two mouse strains carrying these different human APOE genes were shown by comparing mouse melanoma tumor growth as shown in this Figure:
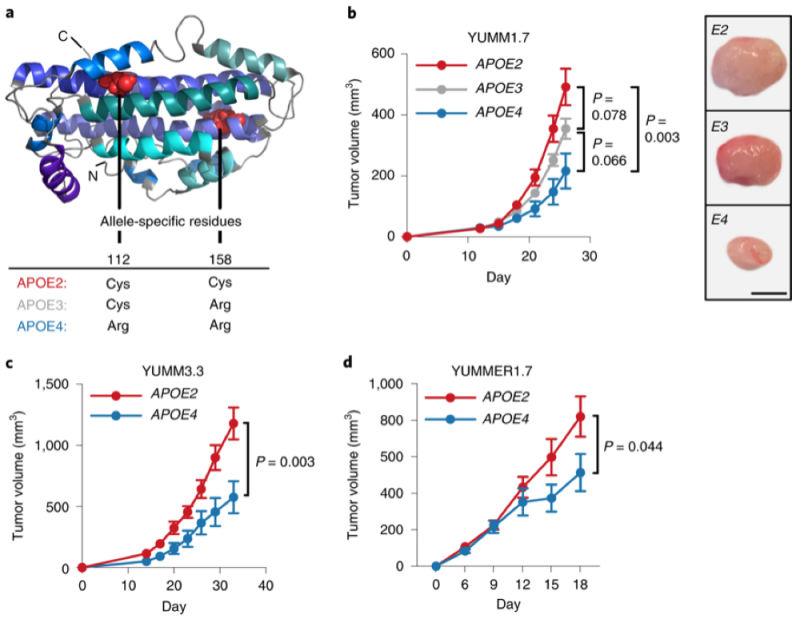
(where YUMM3.3 and YUMMER1.7 are murine melanoma cell lines).
The protective effects against metastasis of APOE4 were compared with APOE2 in these mice was demonstrated by tail vein injection of B16F10 melanoma cells, an established metastasis animal model. The human APOE4-bearing mice had a phenotype of enhanced anti-tumor immune response and improved outcomes under PD1 immune checkpoint blockade.
Because APOE was known to have modulatory effects on immune response, flow cytometric analysis of APOE gene variants in mice was performed and showed "enhanced recruitment of CD45+ leukocytes in animals bearing various melanoma tumors in APOE4 mice compared with APOE2 mice." Proportions of immune suppressor cells (Ly6G+ granulocytic myeloid-derived suppressor cells) were found to be diminished in APOE4-bearing mice, while these mice showed increased proportions of antitumor effector cells such as natural killer (NK) cells and CD8+ T cells. These results were confirmed by single-cell RNA sequencing for detecting lineage-specific gene expression. Further experiments showed that T cell depletion "completely abrogated" differences in melanoma tumor growth between human APOE4- and human APOE2-bearing mice. The authors concluded that "[t]hese data suggest that APOE genotype modulated both the abundance and the functional state of the tumor immune microenvironment, with the APOE4 variant eliciting an enhanced anti-tumor immune profile relative to the APOE2 variant." These authors also showed that APOE4 suppressed melanoma cell invasion and endothelial recruitment (involved in angiogenesis), which was consistent with lower blood vessel density in APOE4 mice.
In addition to these mouse studies, the authors assessed APOE genotype association in melanoma human patients from The Cancer Genome Atlas (TCGA). Neither of these APOE variants was enriched in the database, which the authors said indicated neither gene was involved in increased melanoma incidence. However, APEO4 carriers had improved survival, with 10.1 years for these patients versus 2.1 years for APEO2 carriers. This outcome was surprising due to the reduced longevity associated with APOE4 carriers, which the authors attributed to the high rates of melanoma-associated death. These results demonstrated that "germline genetic variants of APOE differentially associated with survival in patients with advanced melanoma who were at increased risk for melanoma-associated death and metastasis."
PD-1 immunotherapy is a commonly used treatment for melanoma, and "APOE4 mice survived significantly longer than APOE2 mice upon anti-PD1 treatment, suggesting that APOE genotype modulates melanoma outcome also in the context of immunotherapy," according to the results shown in the paper. In humans, "APOE4 and APOE2 carriers exhibited the longest and shortest survival outcomes, respectively, upon anti-PD1 therapy," consistent with the results in mice.
The final set of experimental results reported in this paper involved pharmacologic activation of APOE through liver X receptors, which are "nuclear hormone receptors that transcriptionally activate several genes implicated in cholesterol and lipid metabolism, including APOE." In mice, this effect was completely abrogated in APOE2 mice but showed "robust anti-tumor effects" upon treatment in APOE4 mice. The authors concluded from these results that "distinct APOE genotypes elicited differential responsiveness to LXR agonistic therapy and might serve as potential genetic biomarkers for current clinical efforts investigating the use of LXR agonism in cancer therapy."
The authors provide the following context for the results set forth in their paper:
Our findings have several potential clinical implications. Most importantly, they suggest that common germline variants might serve as biomarkers to identify patients with melanoma who are at high risk for metastatic relapse and melanoma-associated death for treatment with adjuvant systemic therapy. Notably, these clinical association findings will need to be assessed in prospective studies. It will be important to also assess the effect of APOE genotype on the outcome of additional cancer types. More generally, our findings support the notion that hereditary germline variants in the same gene can positively or negatively affect future progression and survival outcomes and responsiveness to therapy in a common human malignancy.
Authors: Benjamin N. Ostendorfa, Jana Bilanovica, Nneoma Adakua, Kimia N. Tafreshiana, Bernardo Tavoraa, Roger D. Vaughanb & Sohail F. Tavazoiea
a Laboratory of Systems Cancer Biology, The Rockefeller University, New York, NY, USA
b Department of Biostatistics, The Rockefeller University, New York, NY, USA