
Mistakes happen; there is even a book, entitled Human Error, that discusses how and why they happen. The Federal Circuit addressed the consequences of human error (or perhaps more accurately, instances where there was a less-than-perfect understanding of the chemical structure of a claimed invention) in a surprisingly lenient fashion in Cubist Pharmaceuticals, Inc. v. Hospira Inc. And the reasoning behind that answer harkens back to the time when the technology for determining structure was not as robust, and when biological molecules comprising chains of amino acids were not routinely defined (and disclosed) by their sequence but rather by how they were isolated or otherwise produced.
The decision arose from ANDA litigation involving daptomycin (sold by Cubist as Cubicin), an antibacterial agent originally developed by Eli Lilly & Co. where the patent on the molecule per se had expired long ago (2002). There were five patents in suit: one patent relating to formulations, compositions, and methods of treating bacterial infections (U.S. Patent No. 5,912,226, now U.S. Reissue Patent No. RE39,071); two patents directed to dosage regimens (U.S. Patent Nos. 6,852,689 and 6,468,967); and two patents directed to purification methods for daptomycin compositions (U.S. Patent Nos. 8,058,238 and 8,129,342).
The District Court held all of the claims in four of the patents to be invalid for obviousness (and a smaller subset invalid for anticipation), but found Hospira's proposed generic daptomycin product to infringe claims 18 (directed to a combination of certain forms of the molecule) and 26 (directed to pharmaceutical compositions of the combinations claimed in claim 18) of the '071 reissue patent and that these claims were not invalid. The combination comprise two daptomycin-related compounds (anhydrodaptomycin and the beta isomer of daptomycin) and the third being daptomycin itself.
The Federal Circuit affirmed in an opinion by Judge Bryson, joined by Judges Wallach and Hughes. Hospira advanced three arguments on appeal related to its invalidity contentions. The first was that the U.S. Patent and Trademark Office erred when it issued a certificate of correction concerning the chemical structure of the daptomycin molecule:
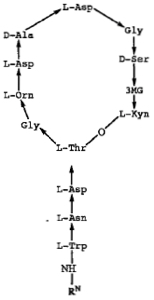
The error concerned the L-Asn (L-asparagine) residue on the amino terminal stem as shown in the diagram. The original specification contained three descriptions of daptomycin as recited in these combinations. The first was "an A-21978C cyclic peptide," identifying the compound as being prepared from "a group of closely related, acidic peptide antibiotics" that are described in U.S. Patent No. 4,208,403"; these peptides are disclosed as being produced by fermentation of Streptomyces roseosporus. The second mode of describing daptomycin in the specification was by a code name assigned by Lilly that was known in the art as being specific for daptomycin. The third way was by the structure shown above, wherein the RN group at the amino terminus was n-decanoyl.
"As it turns out," as stated in the opinion, this structure is incorrect: daptomycin comprises d-Asn, not l-Asn. However, "[a]t the time the application for the '226 patent was filed, and until well after that patent was issued, it was universally believed that the asparagine amino acid in daptomycin was the L-isomer of asparagine, as set forth in the structural diagram," and it was only years afterwards (after the '226 patent was granted and after the '071 reissue patent was filed) that the true stereochemistry of the molecule was discovered. With regard to the patent, Cubist filed and was granted a Certificate of Correction under 35 U.S.C. § 255. That provision of the patent statute permits changes "of minor character" that do not broaden the scope of the claims, and Hospira challenged this characterization of the correction. Hospira supported this argument with expert testimony, to the effect that the molecule comprised of d-Asn was "an entirely different compound" than daptomycin comprising l-Asn (albeit without any reference to the teachings in the '071 patent specification). Accordingly Hospira argued that the District Court should have construed claims 18 and 26 of the '071 patent as being limited to the l-Asn-containing forms of daptomycin (which their proposed generic formulations would not infringe). The District Court disagreed, saying that the change was merely to the formula (i.e., the depiction of the compound's structure) and not any change in the compound itself. The Federal Circuit agreed, citing Regents of Univ. of N.M. v. Knight, 321 F.3d 1111, 1122 (Fed. Cir. 2003), for the proposition that "a chemical structure is 'simply a means of describing a compound; it is not the invention itself'" and further that "the specification as a whole must be considered" not the chemical structure alone. The crux of this reasoning is set forth in this portion of the opinion:
By reference to a co-pending application (later issued as U.S. Patent No. 4,885,243), the specification teaches that daptomycin is obtained through fermentation of Streptomyces roseosporus. That fermentation process necessarily results in daptomycin, not the variant with the L-isomer of asparagine. The evidence at trial established that the L-isomer variant cannot be produced by fermentation and can only be produced synthetically.
The panel also considered the consistent disclosure in the specification identifying daptomycin by its Lilly identification number, and recognized that "it was universally believed that the asparagine amino acid in daptomycin was the L-isomer of asparagine, not the D-isomer" at the time the application was originally filed. In reaching this conclusion the panel distinguished earlier precedent, including Bayer v. Dow Agrosciences LLC, 728 F.3d 1324 (Fed. Cir. 2013). The distinction is that here the description in the specification was consistent with the understanding in the art, whereas in Bayer the proposed claim construction was "inconsistent with the 'strong accepted scientific meaning' of the claim language."
Hospira's second argument was that the specification of the '071 patent did not satisfy the written description requirement due to the error in misidentifying the daptomycin structure. The District Court agreed that the specification "reasonably conveyed to those skilled in the art that the inventors had possession of the claimed subject matter as of the filing date" as required by the statute under Ariad Pharm., Inc. v. Eli Lilly & Co., 598 F.3d 1336, 1351 (Fed. Cir. 2010) (en banc). Despite the error in the disclosed structure, "one skilled in the art would have understood that the inventors possessed and were working with the naturally occurring fermentation product, i.e., the daptomycin molecule containing D-asparagine." The correspondence between the other ways daptomycin was identified in the specification (the Lilly identification code and as being a ""A21978C cyclic peptide") with the product of S. roseosporus fermentation established for the Federal Circuit panel that the skilled worker would have understood the inventors to have been in possession of daptomycin despite the error in chemical structure, citing Invitrogen Corp. v. Clontech Labs., Inc., 429 F.3d 1052, 1072 (Fed. Cir. 2005). And the Court rejected Hospira's assertion that their position was supported by the Court's earlier decision in In re Wallach, 378 F.3d 1330 (Fed. Cir. 2004). In that case, the inventors were in possession of only 5% of the amino acid sequence of a particular protein (although they were in possession of an isolate of the protein itself) and attempted to use that disclosure to support claims to all nucleic acids encoding the entire protein. The panel quotes the distinction made in Wallach (and relevant to the broader issues discussed here) that "whether the applicants 'were in possession of the protein says nothing about whether they were in possession of the protein's amino acid sequence'" because there was no evidence that "the full amino acid sequence of a protein can be deduced from a partial sequence and the limited additional physical characteristics" identified and disclosed in the Wallach specification.
Hospira's last argument was that Cubist had violated the "recapture rule" of reissue patents, that a patentee cannot reclaim through reissue what had been relinquished during prosecution in order to obtain an allowance. Application of the recapture rule requires that the reissued claims were broader than the claims as originally granted (a position the District Court and Federal Circuit rejected with regard to the certificate of correction issue) and that the broadened subject matter had been surrendered during prosecution of the original application. While this doctrine applies if the reissue claims have been broadened in any way, it does not apply if the claims are narrowed. Here, the Federal Circuit agreed with the District Court that claims 18 and 26 were narrower than those claims as originally granted and thus that the recapture rule was not violated by these claims. (In addition, the decision discussed the course of prosecution to the effect that the subject matter in the reissue claims had not been surrendered during prosecution, because any surrender was related to overcoming an indefiniteness rejection and not due to any asserted prior art.)
The Federal Circuit also affirmed the District Court's determination that all of the asserted claims of the other four patents in suit were invalid for obviousness, based in large part on its factual determinations regarding the state of the prior art and on Cubist's failure to provide sufficient evidence of secondary considerations to overcome Hospira's prima facie case.
The Federal Circuit's decision in this case is reminiscent of the days before the biotechnology revolution provided purified proteins as the result of recombinant genetic methods wherein the amino acid sequence of the produced proteins were predicted from the cloned nucleic acids encoding them. Prior to that, purified proteins were isolated from natural sources (for example, insulin from animal pancreata obtained inter alia from slaughterhouses). Patent law recognized it to be sufficient for an applicant to disclose a reliable source of the desired protein and methods for isolating it in useful quantities. (The reader will be spared any discussion here of whether such claims would be patent eligible.) But a consequence of this regime, mandated by the technology available, was that any "purified" preparation of a protein from a natural source was likely not to be homogeneous. It is well known that animals in an outbred population will have a plurality of alleles of a gene for almost any protein that have small variances in amino acid sequence. The population heterogeneity was recognized but not considered relevant to providing a disclosure that supported claims to the isolated protein preparation. In contrast, producing proteins by recombinant genetic technology imposed a homogeneity on the produced protein product that may by itself be sufficient to distinguish such preparations from preparations made from natural sources. But it also changed (some would contend, raised) the quanta of disclosure needed to satisfy the written description requirement so that undisclosed sequence differences became relevant to claim validity.
In this decision the Federal Circuit eschewed such sequence-dependent reasoning and relied instead on the totality of what the specification disclosed. The decision was based in large part on the identity of what S. roseosporus fermentation produced, and the irrelevance (for the panel) of the erroneous structure contained in the specification (and claims). This isn't a right-or-wrong approach; rather, it relies as many patents granted in the decades prior to biotechnology did on identifying a reliable source of a natural product and a description of what the specification provided. Which, even in the biotechnology era, seems a refreshing application of sound patent law.
Cubist Pharmaceuticals, Inc. v. Hospira Inc. (Fed. Cir. 2015)
Panel: Circuit Judges Wallach, Bryson, and Hughes
Opinion by Circuit Judge Bryson