Late last week, the U.S. Food and Drug Administration issued a final version of its "Guidance for Industry," entitled "Considerations in Demonstrating Interchangeability With a Reference Product" regarding the as-yet unexercised provision of the Biologic Price Competition and Innovation Act (BPCIA). This Guidance is facially similar to the draft Guidance issued this past January for notice and comment, and thus reflects the agency's response to those comments (see "FDA Issues Guidance Regarding Interchangeability of Biosimilar and Biologic Drugs").
To recap, interchangeability, which is the standard for conventional, small molecule generic drugs, is challenging for biologic drugs because of their size and complexity, and because the biosimilarity standard encompasses molecules that are not atom-for-atom identical to the reference biologic drug product. Accordingly, the ease with which conventional generic drugs are substituted for brand name versions is not appropriate for biosimilar drugs, and the statute sets out standards for interchangeability status (§ 351(k)(4) of the PHS Act), wherein the FDA must find that:
(A) the biological product—
(i) is biosimilar to the reference product; and
(ii) can be expected to produce the same clinical result as the reference product in any given patient; and
(B) for a biological product that is administered more than once to an individual, the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product without such alternation or switch.
Interchangeability is important for a variety of reasons, particularly with regard to biosimilar drug acceptance and the ability for the interchangeable biosimilar to be substituted for a reference biologic drug product without intervention or approval of a health care provider. Accordingly, such drugs are rewarded with additional layers of exclusivity (indeed, the only exclusivity for biosimilar drugs contained in the statute, as set forth in the PHSA under § 351(k)(6)), wherein the FDA shall not grant interchangeability status for any second biosimilar drug until the later of:
(A) 1 year after the first commercial marketing of the first interchangeable biosimilar biological product to be approved as interchangeable for that reference product; [or]
(B) 18 months after –
(i) a final court decision on all patents in suit in an action instituted under subsection (l)(6) against the applicant that submitted the application for the first approved interchangeable biosimilar biological product; or
(ii) the dismissal with or without prejudice of an action instituted under subsection (l)(6) against the applicant that submitted the application for the first approved interchangeable biosimilar biological product; or
(C)(i) 42 months after approval of the first interchangeable biosimilar biological product if the applicant that submitted such application has been sued under subsection (l)(6) and such litigation is still ongoing within such 42-month period; or
(ii) 18 months after approval of the first interchangeable biosimilar biological product if the applicant that submitted such application has not been sued under subsection (l)(6).
The Guidance provides the FDA's attempt to operationalize the statutory requirements and give the biopharmaceutical industry guidance on what the agency will require to grant interchangeability status to a biosimilar drug.
Like the draft Guidance, this final version of the Guidance is expressly focused on therapeutic protein products with regard to the evidence necessary to establish interchangeability with a reference biologic drug product that is a therapeutic protein. As with almost all other FDA Guidances on biosimilars, this one recites that interchangeability determinations shall be made after consideration of the totality of the evidence that a biosimilar dug satisfies the statutory requirements. Thus, the first requirement is that a biosimilar applicant show that its drug is biosimilar to the reference biologic drug product, and envisions that first licensure will be on biosimilarity grounds. With regard to the requirement that a purportedly interchangeable biosimilar drug would be "expected to produce the same clinical result as the reference product in all of the reference product's licensed conditions of use," the Guidance sets forth a nonlimiting set of data and information:
• The identification and analysis of the critical quality attributes
• The identification of analytical differences between the reference product and the proposed interchangeable product, and, in addition, an analysis of the potential clinical impact of the differences
• An analysis of mechanism(s) of action in each condition of use for which the reference product is licensed, which may include the following:
- The target receptor(s) for each relevant activity/function of the product
- The binding, dose/concentration response, and pattern of molecular signaling upon engagement of target receptor(s)
- The relationship between product structure and target/receptor interactions
- The location and expression of target receptor(s)
• The pharmacokinetics and biodistribution of the product in different patient populations
• The immunogenicity risk of the product in different patient populations
• Differences in expected toxicities in each condition of use and patient population (including whether the expected toxicities are related to the pharmacological activity of the product or to off-target activities)
• Any other factor that may affect the safety or efficacy of the product in each condition of use and patient population for which the reference product is licensed
If there are differences between the reference biologic drug product and the biosimilar with respect to any of these factors, the Guidance asserts that the biosimilar applicant must supply a scientific justification as to why those differences don't preclude a determination of interchangeability. However, the Guidance does not envision that satisfying this standard will necessarily require additional clinical studies. Also, an applicant can (but the FDA recommends that it does not) seek approval for less than all the indications approved for the reference biologic drug product (and in this regard, the Guidance envisions that an applicant may "extrapolate" the data and information supporting interchangeability for one indication to support interchangeability for additional indications).
As for the other statutory requirement, that "for a biological product that is administered more than once to an individual, the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product without such alternation or switch," the Guidance expects a biosimilar applicant will need to do one or more "switching studies" (the general requirements being set forth with some specificity in the Guidance) that will be used to assess the risk to safety or efficacy of alternating between the reference biologic drug product and the biosimilar. Provided as an attachment is a generic description of a switching study design:
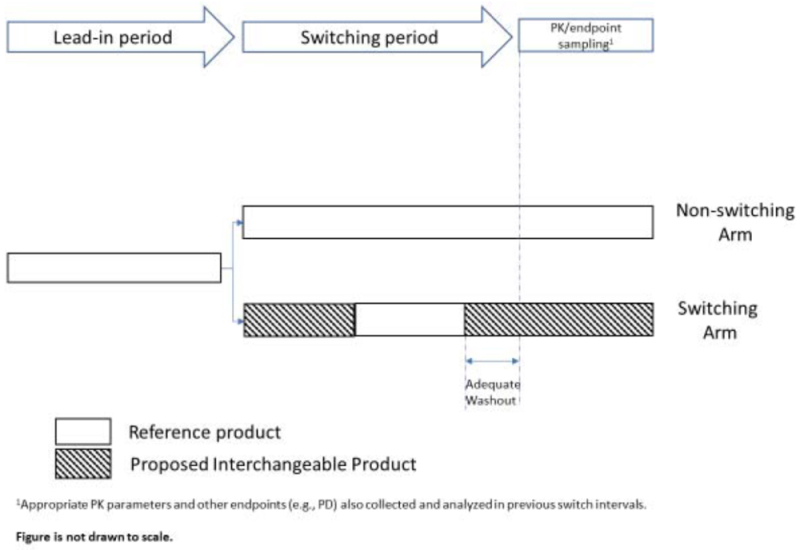
The Guidance provides "an overview of important scientific considerations in demonstrating interchangeability with a reference product," including:
• Data and information needed to support a demonstration of interchangeability
• Considerations for the design and analysis of a switching study or studies to support a demonstration of interchangeability
• Recommendations regarding the use of a U.S.-licensed reference product in a switching study or studies
• Considerations for developing presentations, container closure systems, and delivery device constituent parts for proposed interchangeable products.
And the Guidance then sets forth more detailed explication of these considerations, including product-dependent factors that could influence the data needed to show interchangeability (again urging a "stepwise" approach to establishing interchangeability as the FDA has urged for establishing biosimilarity) and showing a high degree of biosimilarity (with reference to biosimilarity Guidances regarding so-called "fingerprint identity" measures of biosimilarity). The information and data should be aimed at reducing the amount of residual uncertainty, which is expected to depend on the structural and functional complexity of the biosimilar drug. Particularly called out in this regard is the risk of product-specific immunogenicity, which may be more relevant to some biosimilar drugs than others depending on the nature of the reference biologic drug product.
The Guidance also envisions that interchangeability may require biosimilar product postmarketing data, and that such data would not obviate the need for other, interchangeability-related data (e.g., from switching studies, particularly with regard to comparison of pharmacokinetic or pharmacodynamics parameters).
The Guidance provides a detailed discussion of the characteristics of switching studies expected to be required to satisfy interchangeability requirements, for those drugs expected to be administered to a patient more than once (presumably this category encompasses many of not most biologic drugs). These studies will depend on how the drug will be used in practice, according to the Guidance, and the Guidance sets out considerations regarding study endpoints, design and analysis, sample size and number of switches, sampling for PK, PD and immunogenicity, study population, and study analysis, as well as conditions of use and routes of administration.
There is also a section regarding the conditions under which data can be extrapolated (e.g., from one condition of use to another for which the reference biologic drug product is licensed), supported by scientific justification based on mechanism of action, immunogenicity risk, expected toxicities, or "[a]ny other factor that may affect the safety or efficacy of the product in each condition of use and patient population for which the reference product is licensed."
Unlike the Guidances concerning the grounds for establishing biosimilarity, which permit comparative data with a non-U.S. licensed reference biologic drug products to be submitted, the Guidance states that switching studies must be performed using a U.S. licensed reference biologic drug product. This is because in these studies the reference biologic drug product is not used just as a control but is also part of the study, administered in both the switching arm and the non-switching arm. This limitation appears to be based on concerns regarding unpredictable differences in immunogenicity or PK profiles, as well as the existence of several ex-U.S. versions of biosimilar drugs having slight but perhaps clinically relevant differences when used in a switching study that could negatively impact the reliability of study results.
The Guidance ends with a detailed description of presentation designs for the data and information supporting an interchangeability determination (referencing Section VII of the FDA's earlier Guidance entitled Scientific Considerations in Demonstrating Biosimilarity to a Reference Product) and a section on postmarketing safety monitoring.
Despite its status as being "Final," this Guidance, like all such Guidances, contains an express disclaimer:
This guidance represents the current thinking of the Food and Drug Administration (FDA or Agency) on this topic. It does not establish any rights for any person and is not binding on FDA or the public. You can use an alternative approach if it satisfies the requirements of the applicable statutes and regulations. To discuss an alternative approach, contact the FDA office responsible for this guidance as listed on the title page.
In a press release accompanying release of this Guidance, Acting FDA Commissioner Ned Sharpless, M.D. provides some insight into the need for and timing of this Guidance. In addition to generic statements about the economic desirability of biosimilars to provide lower cost access to biologic drugs (recognized as being more costly that conventional, small molecule drugs), Acting Commissioner Sharpless states that:
The final interchangeability guidance is informed by the FDA's cumulative experience providing development-stage advice to sponsors of proposed interchangeable products. The FDA meets regularly with sponsors of proposed interchangeable products through the agency's Biosimilar Product Development Program. The agency also considered the numerous comments on the draft interchangeability guidance and made changes to provide increased clarity to stakeholders. Our rigorous scientific standards for approval will be maintained for interchangeable biologics and should serve as assurance to health care professionals and patients that they can be confident in the safety and effectiveness of both interchangeable products and biosimilar products, just as they would be for reference products.
And in particular:
Separately, and of particular importance to the millions of Americans with diabetes, the final interchangeability guidance will help enable biosimilar or interchangeable insulin products to come to market in the future. There are currently no approved insulin products that can be substituted at the pharmacy level. But, under the BPCI Act, on March 23, 2020, insulin and other biological products that were approved as drugs under the Federal Food, Drug, and Cosmetic Act will be deemed biological products licensed and regulated under the PHS Act. After this transition, the FDA will be able to license biosimilar and interchangeable insulin products that meet the requirements of the PHS Act, and today's guidance will, among other things, help developers seek licensure for such products.
An interchangeable insulin product may be substituted at the pharmacy, potentially leading to increased access and lower costs for patients. For chronically used biologic medications patients get at the pharmacy, such as insulin, the ability to have a licensed interchangeable that can be substituted at the pharmacy without the intervention of the prescribing health care professional -- much like how generic drugs are routinely substituted for brand name drugs -- could be integral to the success of reducing drug prices for patients.
In view of the political firestorm that has arisen over the increased cost of insulin, the FDA's timing of this guidance seems directly applicable to the political response by the Trump administration. This impression is bolstered by Acting Commissioner Sharpless's further comments, that "the agency [intends to] hear from patients, advocates and industry about what factors the agency should consider when evaluating data and other information submitted by an applicant, including from analytical and clinical studies, to determine whether an insulin product is biosimilar to or interchangeable with a reference product" and that the agency "expect[s] to hear stakeholder feedback on whether certain insulin products -- for example, those that use insulin pumps for continuous subcutaneous infusion among the approved uses -- raise unique scientific considerations that we should be considering when evaluating biosimilar or interchangeable insulin products." More ominously for the industry, the Acting Commissioner states that "we'll also be seeking input directly from patients about their experience with insulin products and this input will inform the FDA's approach to implementing the regulatory pathway for biosimilar and interchangeable insulin products." It is rare but perhaps welcome that an agency official so transparently informs stakeholders of both the government's intentions and the information it will consider in determining a regulatory regime for (in this case) reducing particular drug prices, but in the current political climate and with this Administration, perhaps it is not that surprising.