When it comes to SARS-CoV-2 infection (and resulting COVID-19), it seems our Neanderthal ancestors giveth and taketh away. Genetic material inherited from interbreeding between Neanderthals and early humans has been shown to increase the risk of serious COVID infection (see "Inherited Neanderthal Gene Encodes Genetic Risk for COVID-19"). Recently, further explication of correlations between Neanderthal remnants in genomic DNA of modern humans has shown that a sequence on chromosome 12 is associated with milder COVID disease.
These results were reported by the same researchers who reported the earlier, more sinister connection between Neanderthal DNA and COVID-19, which included Svante Pääbo, who almost single-handedly created the science of detecting Neanderthal DNA in archeological samples (and living humans; see Neanderthal Man: In Search of Lost Genomes). In this more recent study, published in the Proceedings of the National Academy of Sciences USA, and entitled "A genomic region associated with protection against severe COVID-19 is inherited from Neandertals," it is a region of chromosome 12 that contains the relevant, seemingly protective genetic locus. Humans bearing this haplotype have a 22% reduction in risk of severe COVID infection. It is present "at substantial frequencies in all regions of the world outside Africa," a hallmark of Neanderthal DNA which arose after humans migrated from the place of our initial evolutionary development. Unlike the earlier, deleterious locus on chromosome 3, for which there is no known function, the region of chromosome 12 associated with milder COVID infection is known to encode proteins that are expressed "during infections with RNA viruses" according to these authors. Another unique inheritance pattern is that this locus appears to have increased in frequency between 20,000 and 10,000 years ago and again approximately 1,000 years ago, which suggests selection that could have been very effective at times when effective medical intervention would have been practically non-existent.
With regard to the function of this locus in Neanderthals themselves, the authors posit that these early humans would have needed to adapt to environments outside Africa which undoubtedly would have included new pathogens that cause infectious disease. Such a pattern of genetic variants that affect genes involved in innate immunity have been shown to have been introduced into Homo sapiens sapiens ancestors from Neanderthal and Denisovan populations, including for example genes that reduce susceptibility to H. pylori infection.
The current study involved genetic assessment of 2,244 terminally ill COVID 19 patients as part of the Genetic of Mortality in Critical Care (GenOMICC) consortium. In addition to the two loci that have been discussed previously and herein, genetic loci relevant to COVID-19 infection have also been found on chromosomes 6, 19, and 21. Specifically with regard to the chromosome 12 locus, these researchers reported association of single nucleotide polymorphisms (SNPs) with this relevant chromosomal loci; this locus satisfied the further criterion of being absent from the genomes of 108 Yoruba individuals (the loci on chromosome 6, 19, and 21 failed this characterization test). The chromosome 12 SNPs were found to be in linkage disequilibrium (i.e., are separated at meiosis at lower than expected frequencies) and formed a ~75kb haplotype. The researchers interpreted this inheritance pattern to be consistent with introduction of these sequences into the modern human gene pool by interbreeding with Neanderthals. Further genetic analyses informed these researchers that this region was not a remnant of DNA inherited by both Neanderthals and modern humans from the time (~550,000 years ago) they diverged but rather were more consistent with mixing when the two populations co-habited beginning ~100,000 years ago. This locus is also present in the three authentic Neanderthal genomes available in current databases.
The geographic distribution of this locus in modern human populations is illustrated by this figure,
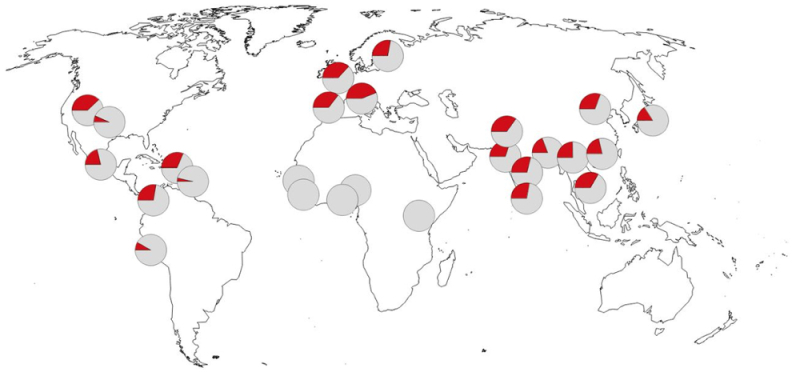 which shows the veritable absence in Africa and variable frequencies (25-30%) around most of the rest of the world.
which shows the veritable absence in Africa and variable frequencies (25-30%) around most of the rest of the world.
The researchers found three genes (OAS1, OAS2, and OAS3) that encode oligoadenylate synthases present at this Neanderthal-derived locus. These genes in modern humans are induced by interferon produced by RNA virus infection and which produce a product (short-chain polyadenylates) that activate a double-stranded RNA specific nuclease. Other antiviral responses are induced as well as a consequence of this generic induction, suggesting a mechanism for resistance to RNA virus infection. Finer scale genetic mapping of SNPs and COVID severity protection implicated OAS3 (although these authors state that this association is "tenuous"). Also of interest is a SNP in the OAS1 gene which alters splicing of the encoded mRNA transcript and produces several isoforms of the encoded protein in its modern human counterpart. Compared with these isoforms, the researchers reported that the ancestral Neanderthal protein has higher enzymatic activity. This isoform is also present in African populations outside this locus, which these authors posit was an ancestral form lost in humans during the time they left Africa. Functionally, this splice site variant has been associated in earlier studies with protection against West Nile Virus. The Neanderthal-derived locus also has missense variants in the OAS1 and OAS2 genes, and two synonymous variants in the OAS3 gene, but these are a mixture of variants present in ancestral African DNA and later acquired in Neanderthal DNA. The missense variant in the OAS1 gene has been previously associated with moderate-to-strong protection against SARS-CoV infection, although these researchers could not rule out the possibility that the responsible genetic factor was merely closely linked to this gene. Finally, this Neanderthal locus on chromosome 12 also seems to provide increased resistance to Hepatitis C infection.
The authors conclude by noting the suggestion that this Neanderthal-derived DNA, particularly the OAS loci contained therein, had undergone positive selection over the past 1,000 years and their hope that "[f]uture studies of human remains from historical times will clarify whether, and when, this occurred."