The "Obvious to Try" Test Is Overused in Assessing Obviousness
The UK Court of Appeal Decision in Novartis AG vs Generics (UK) Ltd (trading as  Mylan), provides insight into the UK court's approach to judging the validity of patents relating to enantiomers of biologically active compounds. UK Patent No. 2,203,040 to Novartis AG (and the associated Supplementary Protection Certificate (SPC)) protected rivastigmine, a drug used for the treatment of Alzheimer's disease. The Court of Appeal held the patent to be invalid on the ground of obviousness and criticised the over-reliance on the "obvious to try" test for assessing obviousness.
Mylan), provides insight into the UK court's approach to judging the validity of patents relating to enantiomers of biologically active compounds. UK Patent No. 2,203,040 to Novartis AG (and the associated Supplementary Protection Certificate (SPC)) protected rivastigmine, a drug used for the treatment of Alzheimer's disease. The Court of Appeal held the patent to be invalid on the ground of obviousness and criticised the over-reliance on the "obvious to try" test for assessing obviousness.
The Patent
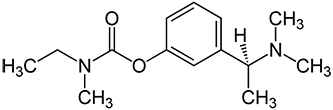 A chiral compound exists as two or more non-superimposable, mirror-image chemical structures, the alternative structures being known as enantiomers. Rivastigmine is the (-)enantiomer of the chiral carbamate compound, N-ethyl-3-[(1-dimethylamino)ethyl]-N-methylphenyl-carbamate. UK Patent No. 2,203,040 claims rivastigmine. The compound is marketed in the UK by Novartis as Exelon®, and is used for the treatment of Alzheimer's disease.
A chiral compound exists as two or more non-superimposable, mirror-image chemical structures, the alternative structures being known as enantiomers. Rivastigmine is the (-)enantiomer of the chiral carbamate compound, N-ethyl-3-[(1-dimethylamino)ethyl]-N-methylphenyl-carbamate. UK Patent No. 2,203,040 claims rivastigmine. The compound is marketed in the UK by Novartis as Exelon®, and is used for the treatment of Alzheimer's disease.
The High Court Decision
The High Court assessed whether it would have been obvious to a skilled person to select RA7, resolve it into its individual enantiomers, and then use the (-) enantiomer as a medicinal product for the treatment of Alzheimer's disease.
A racemic mixture (RA7), a mixture of the (+) and (-) enantiomers of N-ethyl-3-[(1-dimethylamino)ethyl]-N-methylphenyl-carbamate, was known at the priority date of the Patent and had been identified as a potential treatment for Alzheimer's disease.
The UK High Court therefore held that it would have been obvious to select the racemic mixture, RA7, to resolve it into its individual enantiomers, and to use the claimed (-) enantiomer for the treatment of Alzheimer's disease. The Patent and associated SPC were therefore found to be invalid.
The Court of Appeal Decision
 On appeal, Novartis argued that the High Court erred in its assessment of obviousness. Novartis argued that that it would not have been "obvious to try" and resolve RA7 into the individual enantiomers, nor would there have been any expectation that the claimed (-) enantiomer would possess improved biological activity compared to the (+) enantiomer.
On appeal, Novartis argued that the High Court erred in its assessment of obviousness. Novartis argued that that it would not have been "obvious to try" and resolve RA7 into the individual enantiomers, nor would there have been any expectation that the claimed (-) enantiomer would possess improved biological activity compared to the (+) enantiomer.
The Court of Appeal criticised over-reliance on the obvious to try test in assessing inventive step. The Court of Appeal acknowledged that it might be appropriate when assessing inventive step to ask whether it was obvious to try a particular route with a reasonable expectation of success. However, the Decision emphasized that such assessment is secondary to the statutory question of whether an invention is obvious:
This is another case in which a patentee defending his patent has attempted to analyse a single multi-faceted question ("was the invention obvious?") by chopping it up into a series of sub-questions, and then treating each of the sub-questions in isolation. . . . [T]hat is the wrong approach.
In the present case it was clear that the prior art disclosed a group of compounds, including RA7, that were potentially useful in treating Alzheimer's disease. Biological data published in the prior art also showed that RA7 was an obvious candidate for further development. The skilled person was also well aware of the common practice at the priority date to attempt to resolve racemic compounds at an early stage of the drug development process and that there would likely be practical benefits associated with such resolution.
On this evidence the Court of Appeal upheld the judgement of the High Court and the patent was held invalid.
Conclusions
Earlier UK Court of Appeal decisions (Generics (UK) Limited v. Daiichi Pharmaceutical Co Ltd and H Lundbeck A/S v. Generics (UK) Limited) held patents relating to the biologically active enantiomers escitalopram and levofloxacin were non-obvious. However, evidence in these cases indicated that there were particular problems with resolving the enantiomers at the relevant priority date. The evidence in the present case indicated that there were no problems encountered in resolving RA7 and that resolution could be achieved using conventional techniques.
The present Decision might suggest that obtaining patent protection for single enantiomers in the UK is likely to prove difficult if the enantiomer was easily resolved from a known racemic mixture. If data can be produced that demonstrate practical difficulties with isolating an enantiomer from a racemic mixture or an unexpected therapeutic effect associated with the claimed enantiomer then objections of obviousness may be overcome.
This report comes from European Patent Attorneys at WP Thompson & Co., 55 Drury Lane, London UK. Further details and commentary can be obtained from Gill Smaggasgale, a partner at the firm.