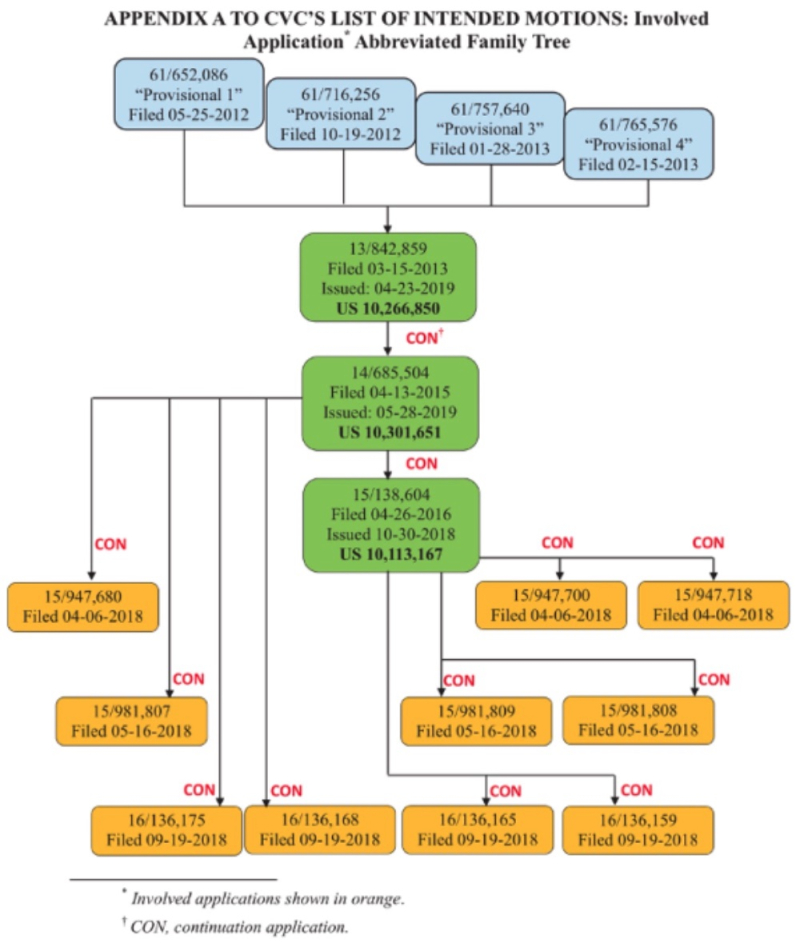
To recap, CVC argued that "CVC invented a eukaryotic cell comprising a single-molecule guide RNA ("sgRNA") CRISPR-Cas9 system capable of cleaving or editing target DNA, as defined by the count" to define the issue, and then gives the Board a basis for coming to a different conclusion here. Specifically, CVC argues that (as a result of the '115 Interference) it now has additional evidence in support of this motion. (CVC made a similar argument in the '127 Interference.)
Most of CVC's arguments are familiar to anyone following parallel motions in the '115 and '127 interferences. These include CVC's contention that once this breakthrough had been achieved, adapting CRISPR to the eukaryotic cell environment would have been "pretty straightforward" (quoting Dr. Luciano Marraffini, who purportedly informed the Broad inventors of the sgRNA embodiment in June, 2012 (see "CVC Files Motion in Opposition to Broad Priority Motion"). CVC supported this assertion with contemporaneous consistent statements from Rodolphe Barrangou, Erik Sontheimer, Samuel Sternberg, and Dana Carroll, as well as Jennifer Doudna; by the existence of "existing platforms that had already been successfully used with the two incumbent systems: zinc-finger nucleases ("ZFNs") and transcription activator-like effector nucleases ("TALENs")"; and by the successful practice of CRISPR by several groups (including Sigma-Aldrich) "[j]ust months after CVC presented this work" and the absence in the reports from any of these groups of "any 'special' adaptations or conditions needed" to achieve CRISPR gene editing in eukaryotic cells. And CVC argued that the Board's contrary conclusion in denying CVC's motion for priority benefit to the P1 and P2 provisional applications in the '115 Interference was that it was made "without the benefit of the now well-developed evidentiary record," specifically, that "[t]he prior decision credited assertions that have been seriously undermined by evidence presented during the priority phase of the '115 interference." That evidence was presented in CVC's Motion, which will not be recapitulated here.
Sigma-Aldrich's Opposition countered CVC's assertions using the same approach successfully used by Broad in persuading the Board to find priority of invention in their favor in the '115 interference (see "PTAB Holds for Broad in CRISPR Interference: The Reasoning")*: that the two provisional applications (filed on May 25, 2012 and October 19, 2012) did not disclose an operative embodiment of CRISPR that could be successfully practiced in eukaryotic cells. (Sigma-Aldrich does not challenge CVC's motion with regard to the P3 provisional application, no doubt because inter alia its status as Senior Party in this interference would not change should the Board do so.) Harkening back to the Board's decision on CVC's motion in the '115 interference, Sigma-Aldrich argued that the Board was correct in its prior determination that those applications only disclosed in vitro CRISPR methods in a "cell-free" environment. According to Sigma-Aldrich, nothing has changed that would have the Board render a decision different from their refusal in the '115 interference to accord CVC benefit to P1 and P2 provisional application, and the Board should come to the same conclusion (the brief noting that CVC has the burden as the party advancing the motion to convince the Board to come to a different conclusion here). Because Sigma-Aldrich contends that most if not all the evidence CVC asserts in its brief supporting its priority benefit motion in this interference had been asserted in the corresponding brief in the '115 motion, Sigma-Aldrich contends CVC has not met that burden.
The cornerstone of Sigma-Aldrich's motion is that neither the P1 nor P2 provisional applications disclosed any "specific instructions or conditions" required for the practice of CRISPR in eukaryotic cells and that such instructions and/or conditions were necessary in view of the several obstacles required (tracking the same distinctions between CRISPR in eukaryotic cells and under other conditions recited by Broad in the '115 interference and Interference No. 105,048; see "PTAB Decides CRISPR Interference in Favor of Broad Institute -- Their Reasoning"). Sigma-Aldrich further contends that one basis for CVC's argument is that the ordinarily skilled person would have had a reasonable expectation of success in achieving successful CRISPR in eukaryotic cells in the absence of disclosure in the P1 or P2 provisional applications, in contradiction to the Board's contrary determination in the '048 interference.
In support of its contention that the absence of disclosure of the specific instructions or conditions in the P1 or P2 provisional applications supported its contention that neither disclosed an embodiment within the scope of the Count to satisfy the requirements for priority, Sigma-Aldrich cited Group II introns as analogous embodiments, because:
• Both Group II introns and CRISPR-Cas9 originate in bacteria and function as RNP complexes.
• Both systems use an RNA to interact with a DNA target and to direct the action of the RNP complex.
• The consequences of both CRISPR-Cas9 and Group II introns are sequence-specific changes to prokaryotic DNA that involve endonuclease activities.
And further:
• Group II introns comprise an RNA component and an intron encoded protein.
• LtrA protein encoded by L1.LtrB intron is the best characterized [intron-encoded protein or] IEP . . . . Both LtrA and Cas9 have an HNH endonuclease domain that cleaves a DNA target.
• Group II introns are capable of site-specifically cleaving DNA, or additionally inserting sequences into DNA.
• Because of this, Group II introns have been utilized in prokaryotes for gene targeting applications. This function is mediated by the RNP particle containing the IEP and an excised intron RNA, with DNA target specificity determined by base pairing of the intron RNA to the DNA target sequence, with additional support from the IEP.
But "[d]espite successful use in prokaryotes for gene targeting, only moderate activity of Group II introns has been obtained in eukaryotic cells" Sigma-Aldrich asserts, citing prior art references. Other systems for DNA editing known in the art, such as zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs) can be distinguished from CRISPR (and expected to act differently in vivo) because these complexes do not require an RNA component for DNA cleavage, Sigma-Aldrich contends. Indeed, Sigma-Aldrich argues, this distinction, the requirement for the sgRNA component of CRISPR recited in the Count, is a reason the skilled person would have considered CRISPR-Cas9 to be unique and not analogous to any prior methods for site-specific DNA cleavage. In addition, Sigma-Aldrich argues that the art did not disclose a set of "common factors and conditions" that would be expected to work with CRISPR because they had worked with other systems for cleaving DNA in eukaryotic cells based on prokaryotic mechanisms.
The brief sets forth all the multiple distinctions between prokaryotic cells and eukaryotic cells used successfully in the '048 and '115 interferences (including structural distinctions with eukaryotic mRNA (caps, poly A); the requirement for proper folding; crowding and lack of chaperones in eukaryotic cells; the packaging by chromatin/histones of eukaryotic genomic DNA; uncertainty regarding the need for PAM sequences in sgRNA; intracellular conditions (including ion concentrations); toxicity, inter alia from dsRNA-triggered interferon production in eukaryotic cells; and non-specific binding). Microinjection, which CVC asserts is an embodiment showing successful eukaryotic CRISPR "would not obviate most technical challenges," because most of the circumstances providing impediments also exist in the microinjected cell. Sigma-Aldrich argues that the existence of these impediments (actual or potential) required disclosure of conditions for addressing them but, on the contrary, CVC's argument was that the skilled artisan could presume eukaryotic CRISPR could be successfully achieved without them.
In support for the deficiencies in the P1 and P2 applications Sigma-Aldrich recites portions of these specifications to illustrate:
Histone proteins are known in the art to bind DNA and form complexes known as nucleosomes. Histones can be modified (e.g., by methylation, acetylation, ubiquitination, phosphorylation) to elicit structural changes in the surrounding DNA, thus controlling the accessibility of potentially large portions of DNA to interacting factors such as transcription factors, polymerases and the like . . . . Thus, a site-directed modifying polypeptide with histone-modifying activity finds use in the site-specific control of DNA structure and can be used to alter the histone modification pattern in a selected region of target DNA. Such methods find use in both research and clinical applications.
And:
In some of the above applications, the subject methods may be employed to induce DNA cleavage and DNA modification in mitotic or post-mitotic cells in vitro and/or ex vivo and/or in vitro (e.g., to produce genetically modified cells that can be reintroduced into an individual). Because the DNA-targeting RNA provide specificity by hybridizing to target DNA, a mitotic and/or post-mitotic cell of interest in the disclosed methods may include a cell from any organism (e.g. a bacterial cell, an archaeal cell, a cell of a single-cell eukaryotic organism, a plant cell, an animal cell, a cell from an invertebrate animal (e.g. fruit fly, cnidarian, echinoderm, nematode, etc.), a cell from a vertebrate animal (e.g., fish, amphibian, reptile, bird, mammal), a cell from a mammal, a cell from a rodent, a cell from a human, etc.). Any type of cell may be of interest (e.g. a stem cell, e.g. an embryonic stem (ES) cell, an induced pluripotent stem (iPS) cell, a germ cell; a somatic cell, e.g. a fibroblast, a hematopoietic cell, a neuron, a muscle cell, a bone cell, a hepatocyte, a pancreatic cell etc.) . . . [emphasis in brief].
These disclosures, according to Sigma-Aldrich, demonstrate that the P1 and P2 applications disclose a "mere wish or plan," citing Centocor Ortho Biotech, Inc. v. Abbott Labs., 636 F.3d 1341, 1348 (Fed. Cir. 2011) and fail to satisfy the requirements for priority.
Again as Broad has done in earlier interferences, Sigma-Aldrich recites statements from CVC's witnesses used to cast doubt their positive assertions in support of CVC's motion. For example, from Dr. Carroll:
What about activity of the system in eukaryotic cells? Both zinc fingers and TALE modules come from natural transcription factors that bind their targets in a chromatin context. This is not true of the CRISPR components. There is no guarantee that Cas9 will work effectively on a chromatin target or that the required DNA–RNA hybrid can be stabilized in that context. This structure may be a substrate for RNA hydrolysis by ribonuclease H and/or FEN1, both of which function in the removal of RNA primers during DNA replication. Only attempts to apply the system in eukaryotes will address these concerns [emphasis in brief].
And Dr. Barranghou:
Although immediate applications of this new tool include customized DNA nicking and/or cleavage in bacteria, there are intriguing possibilities for genome editing and genome engineering of eukaryotes. This will require testing whether crRNA-Cas systems can efficiently cleave chromatin DNA in vivo and be readily transferred into organisms of interest, notably yeast and fungi, but also plants, for crop and agricultural applications, and human cells, for medical purposes. Only the future will tell whether this programmable molecular scalpel can outcompete ZFN and TALEN DNA scissors for precise genomic surgery [emphasis in brief].
And of course the brief cites statements from Jennifer Doudna:
These findings [reported in Jinek 2012] suggested the exciting possibility that Cas9:sgRNA complexes might constitute a simple and versatile RNA-directed system for generating DSBs that could facilitate site-specific genome editing. However, it was not known whether such a bacterial system would function in eukaryotic cells.
Our 2012 [Jinek] paper was a big success, but there was a problem. We weren't sure if CRISPR/Cas9 would work in eukaryotes—plant and animal cells. Unlike bacteria, plant and animal cells have a cell nucleus, and inside, DNA is stored in a tightly wound form, bound in a structure called chromatin [emphasis in brief].
The brief also cites the Board's basis for its decision in the '115 interference:
CVC's arguments fail to persuade us that those of ordinary skill in the art would not have considered specific instructions or conditions for a CRISPR-Cas9 activity in a eukaryotic cell to be necessary. Possession of an innovation is not indicated by the need for optimization to obtain it because "[t]he question is not whether a claimed invention is an obvious variant of that which is disclosed in the specification. Rather, a prior application itself must describe an invention, and do so in sufficient detail that one skilled in the art can clearly conclude that the inventor invented the claimed invention as of the filing date sought," citing Lockwood v. Am. Airlines, Inc., 107 F.3d 1565, 1572 (Fed. Cir. 1997) [emphasis in brief].
Accordingly, Sigma-Aldrich asks the Board to deny CVC's motion.
* Thus, Sigma-Aldrich made these arguments before the Board ruled in the '115 Interference.