 The biggest concern of the biotechnology industry caused by the impending Supreme Court decision in the AMP v. Myriad Genetics case is the threat to existing patents having claims to isolated human DNA (and the DNA from other species) that a negative decision from the Court could raise. Patent protection has been an important component of the success of companies from Genentech to Myriad and many others, and the prospect of a categorical ban (ironic, in view of the Court's anathema for "bright line" rules promulgated by the Federal Circuit) threatens the success paradigm developed over the past 30 years. While the extent of whatever deleterious effects on the progress of the biotechnology arts that the Court may impose by its Myriad decision is still to come, an article in the May edition of Nature Biotechnology suggests that those effects may be ameliorated by the anachronistic aspects of the Myriad case: in many ways this argument is coming 30 years to late, and the future of biotechnology is less dependent on so-called "gene patents" than at any other time in the history of the biotechnology industry.
The biggest concern of the biotechnology industry caused by the impending Supreme Court decision in the AMP v. Myriad Genetics case is the threat to existing patents having claims to isolated human DNA (and the DNA from other species) that a negative decision from the Court could raise. Patent protection has been an important component of the success of companies from Genentech to Myriad and many others, and the prospect of a categorical ban (ironic, in view of the Court's anathema for "bright line" rules promulgated by the Federal Circuit) threatens the success paradigm developed over the past 30 years. While the extent of whatever deleterious effects on the progress of the biotechnology arts that the Court may impose by its Myriad decision is still to come, an article in the May edition of Nature Biotechnology suggests that those effects may be ameliorated by the anachronistic aspects of the Myriad case: in many ways this argument is coming 30 years to late, and the future of biotechnology is less dependent on so-called "gene patents" than at any other time in the history of the biotechnology industry.
The article, "Not quite a myriad of gene patents," by Gregory Graff, Devon Phillips, Zhen Lei, Sooyoung Oh, Carol Nottenburg, and Philip Pardey, provides a survey of existing patents claiming isolated human DNA. After providing a brief history of the Myriad case, the paper provides data on the number of U.S. patents containing different types and extents of isolated human DNA:
• Patents that identify DNA or RNA "anywhere" in the patent document
• Patents that identify DNA or RNA in the claims (providing upper and lower estimates)
• Patents with at least one composition of matter claim directed to isolated DNA or RNA (numbering 15,359 patents according to these authors)
Previously published estimates of the number of such patents claiming human DNA vary widely, with a high of 40,000 from a National Research Council report to a emerging consensus of ~4,000 – 5,000 patents that claims isolated human DNA. The paper also addresses the philosophical and practical consequences that could arise if the Court narrowly answered the Question Presented in a decision limited to human DNA, in contrast to DNA from other species.
The quantitative aspects of the paper involve an analysis of claim language in claims reciting isolated human DNA, thereby identifying 72,052 U.S. patents that "in some way identify or make reference to nucleotide sequences." Of these, the authors report that from 30-39% of these patents (21,870 – 28,410 patents) were "likely to contain or refer to nucleotide sequences in at least one of the claims of the patent." Finally, the paper identifies from this population patents that contain at least one composition of matter claim to an isolated human nucleic acid, which are reported to be 15,359 patents. (As an interesting aside, there were 82,952 method claims in the 72,052 patents that recited human DNA or RNA.) Of these 15,359 patents, 5,936 (39%) "primarily involve" isolated human DNA, with the remainder being directed to isolated DNA molecules from other animals (1,056; 7%); plants (1,847; 12%); microbes (3,228; 21); and synthetic or artificial sequences (3,292; 21%). (Methodologically, the authors considered claims to artificial or synthetic sequences to be "markedly different" from naturally occurring DNA and omitted these patents from further analysis.)
Looking at the timing of patents granted on isolated DNA, the authors report that the number of patents containing such claims peaked in 1999 and has been declining since 2005 (no doubt due to a combination of the more stringent utility requirements imposed by the U.S. PTO in 2001 and the change from isolating DNA encoding a particular protein and isolating human DNA containing an open reading frame as identified by genome sequencing efforts, predominantly related to the Human Genome Project). The proportion of patents claiming isolated human DNA peaked in 2000, where 58% of "gene" patents recited human DNA, and by 2010 this percentage had fallen to 19%.
Of the patents containing claims to isolated DNA that could encompass "naturally occurring" molecules, the authors identified 8,703 that remain in force, with 3,535 (41%) of these patents claiming isolated human DNA and less than half of these patents being related to human medicine (an area about which at least Justice Breyer has evinced a particular concern on the possibility of negative effects of patenting on patient care). The assignee data the authors report are a little surprising, with 65% of the 15,359 patents containing composition of matter claims reciting isolated DNA molecules being owned by private-sector commercial assignees, and only 24% being owned by public-sector (identified as "government, university, nonprofit") assignees (the remainder being owned by some combination of public- and private-sector assignees). The "Top Twenty-five" assignees (and the number of patents assigned to them) are reported to be:
The authors speculate on the reason for the decline in patents claiming isolated nucleic acids, providing some clear correlations (the crash of the tech bubble at the turn of the century, the 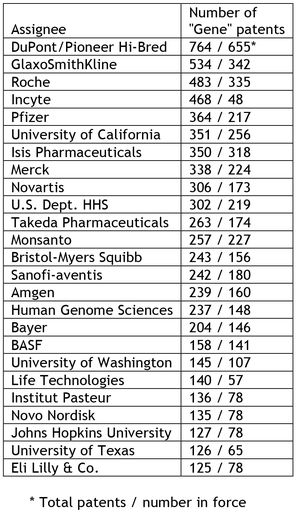 availability on public databases of human genomic DNA sequences and the prior art effect, and the more stringent utility requirements). The authors also recognized that the 1999-2000 timeframe also marked a change from claims to isolated human DNA molecules per se to claims reciting "complex genetic constructs" (presumably including expression constructs). The authors also note the trend over the past decade towards claims to molecules comprising artificial or synthetic sequences (from 14% to 40%) rather than "naturally occurring" DNA molecules (from 58% to 19%). Nevertheless, the 3,535 patents claiming isolated human DNA remain at risk for invalidation to some extent by the Supreme Court's Myriad decision (and, the authors note, the remaining 4,538 patents claiming isolated DNA from other species might also be at risk if the Court rules more broadly). Consequently:
availability on public databases of human genomic DNA sequences and the prior art effect, and the more stringent utility requirements). The authors also recognized that the 1999-2000 timeframe also marked a change from claims to isolated human DNA molecules per se to claims reciting "complex genetic constructs" (presumably including expression constructs). The authors also note the trend over the past decade towards claims to molecules comprising artificial or synthetic sequences (from 14% to 40%) rather than "naturally occurring" DNA molecules (from 58% to 19%). Nevertheless, the 3,535 patents claiming isolated human DNA remain at risk for invalidation to some extent by the Supreme Court's Myriad decision (and, the authors note, the remaining 4,538 patents claiming isolated DNA from other species might also be at risk if the Court rules more broadly). Consequently:
[A]n overturn by the Supreme Court will affect claims of patents not only on human genetic diagnostics and therapeutics but also on a wide range of other genetic technologies in other industries, particularly in agriculture, based upon our analysis of top assignee portfolios.
The authors attempt to find a silver lining in their analyses, particularly with regard to sequence variants and genetically engineered constructs, which may have a greater likelihood to evince "the hand of man" and to be "markedly different" from naturally occurring DNA. They perceive a trend towards this type of innovation that a "product of nature" exception from the Court would "accelerate," and more recent events (the PTO's decision to examine one sequence per invention and the Federal Circuit's expansion of obviousness towards isolated DNA in In re Kubin) are not accounted for in the data. Thus:
The outcome of the Myriad case, regardless, is thus likely to be less profound than either abolitionists or advocates seem to expect. In the end, any policy effects resulting from the Myriad case may prove to have been largely preempted by a combination of less dramatic changes in examination practices and patent law.
While outside the scope of the data presented in their paper, the authors also neglect to consider the effects of a broad "product of nature" ban by the Court on biotechnology more generally (a concern voiced by Judge Moore in oral argument and her concurring opinions in the Federal Circuit's review of the Myriad decision). It is possible, as the authors posit, that the negative effects of whatever decision is handed down by the Court will be ameliorated by advances in DNA technology (and the capacity of competent patent draftsmen to adapt claiming strategies to accommodate their clients' needs in view of the Court's proscriptions). But it is equally likely that the Court will seriously harm the economic incentives for commercializing genetic technology and inhibit disclosure of new correlations between disease and genetic variation, all to the detriment of progress in this industry. And it is a near certainty that the purported purpose behind the controversy, better access to genetic testing for patients, will not be one of the outcomes of any decision that impairs the ability to commercializing genetic testing. In the end, the question is not how many patents are at risk from the Court's consideration of the question before it, but rather why do the Justices not realize that now is not the time to wade unnecessarily into these particular philosophical waters. It is ultimately a political, not a legal, question, and one better suited for consideration by the (expressly) political branches of the government.