Earlier this month, Eric Sagonowsky reviewed the top ten drugs in the U.S. (in terms of sales) losing patent exclusivity in an article published by Fierce Pharma.
These drugs are Lucentis (Genentech/Roche), Bystolic (AbbVie/Allergan), Vascepa (Amarin), Nothera (Lundbeck), Narcan (Emergent Biosolutions), Brovana (Sunovion), Sutent (Pfizer), Saphris (AbbVie/Allergan), Amitiza (Mallinckrodt), and Feraheme (Amag Pharma). The nature of these losses and consequences thereof can be seen from the article, synopsized here.
Lucentis (ranibizumab), Roche's drug for macular degeneration, is a humanized mouse monoclonal antibody fragment specific for vascular endothelial growth factor A. It is related to Roche's Avastin (bevacizumab) product, having been modified for injection into the vitreous humor of the eye for the treatment of wet age-related forms of the disease (AMD).
Bystolic (nebivolol), AbbVie/Allergan's drug for high blood pressure, is a small molecule beta blocker having the formula:
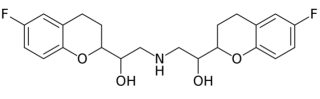
The last patent on this drug product expired on December 17, 2021; under the terms of settlement agreements with several generic competitors, versions of the drug can come on the market (if FDA approved) on September 17, 2021. However, antitrust litigation by direct purchasers may complicate this scenario.
Vascepa (ethyl eicosapentaenoic acid), Amarin's drug for hypertriglyceridemia and cardiovascular disease, is a purified omega-3 fatty acid eicosapentaenoic acid having the formula:
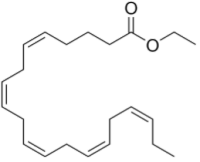
The drug is involved in litigation, Amarin having lost its ANDA litigation action against Hikma Pharmaceuticals (see "Amarin Pharma, Inc. v. Hikma Pharmaceuticals USA Inc. (Fed. Cir. 2020)"), although Amarin brought another lawsuit against Hikma in November 2020.
Nothera (droxidopa), Lundbeck's drug for neurogenic orthostatic hypotension, is a synthetic amino acid analogue that is converted in vivo to norepinephrine and has the formula:
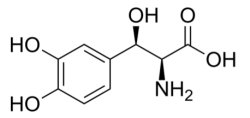
Several generic challengers are in the offing, including Camber Pharma (which launched its generic in February 2021), Hikma (March 2, 2021), and Zydus Cadila (FDA approval May 2020).
Narcan (naloxone) is Emergent Biosolutions's drug known for treating opioid overdose and has the formula:

This drug has been in ANDA litigation with Teva, which received FDA approval in 2019 and invalidated Emergent's Orange Book-listed patents in the district court; that decision is on appeal to the Federal Circuit. Emergent reached a settlement agreement with Perrigo that licenses the company to bring its generic Narcan to market in 2033 or earlier should Emergent be unable to obtain reversal of the district court's decision invalidating its patents.
Brovana (arformoterol) is Sunovion's drug for treating chronic obstructive pulmonary disease (COPD). It is a long-acting β2 adrenoceptor agonist having the formula:

All Sunovion's patents protecting this drug will expire in 2021, and several generic companies have received tentative FDA approval.
Sutent (sunitinib), is marketed by Pfizer for the treatment of cancer including gastrointestinal stromal tumors, advanced renal cell carcinoma, and pancreatic neuroendocrine tumors. It has the formula:
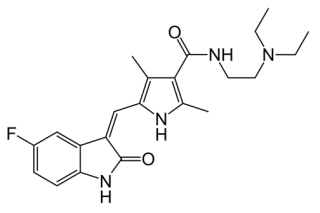
All the drug's patents expire in 2021, and generic competitors include Glenmark and Mylan (which lost an ANDA litigation over its proposed generic version of the drug in 2010).
Saphris (asenapine) is AbbVie/Allergan's drug for treating schizophrenia and bipolar disorder. It has the formula:
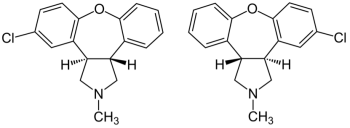
Breckenridge, Alembic, and Sigmapharm launched generic versions of the drug in December 2020.
Amitiza (lubiprostone) is Mallinckrodt's drug for treating constipation and irritable bowel syndrome. It has the formula:
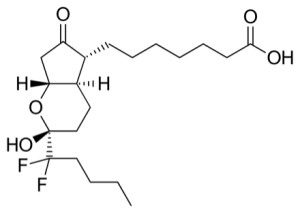
Mallinckrodt recently filed for Chapter 11 bankruptcy.
Feraheme (ferumoxytol) is an intravenously administered, carbohydrate-coated, superparamagnetic iron oxide (Fe3O4) nanoparticle inorganic product for treating anemia marketed by Amag Pharmaceuticals. Under the terms of a patent infringement litigation settlement, Sandoz is authorized to launch its generic version in July 2021 (having received FDA approval in January 2021). Other provisions of this settlement provide for Sandoz to pay royalties to Amag until June 30, 2023 (when the last patent expires) and for Amag to market an authorized generic in July 2022.
The economic consequences for these companies can be assessed by their U.S. sales, although as Mr. Saganosky reports, not all of them will be facing generic or biosimilar competition right away (for various reasons including court battles and regulatory approval delays).
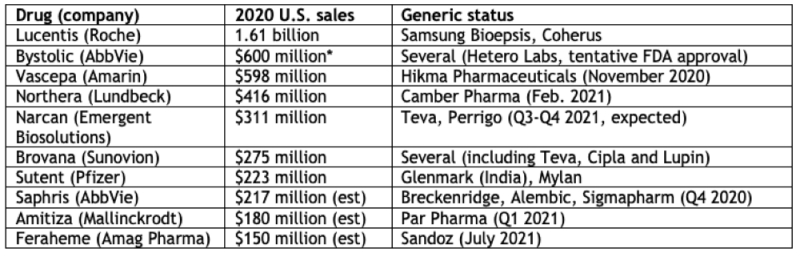 * 2019 U.S. sales data
* 2019 U.S. sales data
Mr. Sagonowsky notes that his report is based on information "from numerous sources, including lists of potential generic launches from OptumRx, GoodRx, GreyB, and Corporate Pharmacy Services, plus company filings, conference calls with analysts, FDA records and more."