The decision by the Patent Trial and Appeal Board (PTAB) in favor of Senior Party the Broad Institute, Harvard University, and MIT (collectively, "Broad") and against Junior Party the University of California/Berkeley, the University of Vienna, and Emmanuelle Charpentier (collectively, "CVC") sixteen months ago in the latest CRISPR interference No. 106,115 is the subject of appeal from both parties. CVC filed its Opening Brief (discussed herein) on September 20, 2022; the remaining briefs will be the subject of later posts.
To recap, the Board was convinced by Broad's arguments that CVC's attempts to reduce eukaryotic CRISPR to practice were unavailing until after Broad's reduction to practice as evidenced by a manuscript submitted on October 5, 2012. Operating on the legal principle that "priority of invention goes to the first party to reduce an invention to practice unless the other party can show that it was the first to conceive of the invention and that it exercised reasonable diligence in later reducing that invention to practice," Cooper v. Goldfarb, 154 F.3d 1321, 1327 (Fed. Cir. 1998), the Board was unconvinced that CVC's March 1, 2012 conception satisfied the requirements of "complete" conception. Using much of the same argument (albeit for different purposes) as it had to prevail in Interference No. 106,048 (see "PTAB Decides CRISPR Interference in Favor of Broad Institute -- Their Reasoning"), the Broad persuasively argued that the evidence of CVC's attempts to reduce eukaryotic CRISPR to practice showed sufficient uncertainty and failures for the Board to conclude that CVC did not satisfy the requirements for conception. On this evidence the Board was unpersuaded that all that had been needed was the application of routine experimentation using the sgRNA detailed in CVC's March 1st priority statement. Nor was the Board convinced that Broad derived the embodiments of eukaryotic CRISPR that they reduced to practice embodying sgRNA only after Dr. Marraffini obtained it from CVC and disclosed it to the Broad inventors (see "CVC Files Reply to Broad's Opposition to CVC's Priority Motion"). And addition to the decision on priority, the Board denied CVC's motion for improper inventorship under 35 U.S.C. § 102(f) (see "CVC Files Substantive Motion No. 3 (for Improper Inventorship) and Broad Opposes") for evidentiary deficiencies, and in their discretion refused to consider CVC's allegations of inequitable conduct against the Broad (see "Inequitable Conduct by Senior Party Broad Alleged in Interference No. 106,115 (and PTAB May Finally Hear Evidence About It)").
CVC's brief begins with a reminder that Drs. Doudna and Charpentier had received the Nobel Prize for CRISPR (as every paper filed in the '115 Interference has done since the award). The brief also sets forth the timeline of CVC's conception and reduction to practice, containing several relevant considerations for the Federal Circuit to consider when assessing whether the Board erred in reaching its decision. These include (first and foremost) that Broad and colleagues derived their invention totally from CVC's public disclosure of sgRNA species; according to CVC everything else was just routine applications of introducing exogenous molecules into eukaryotic cells using methods well known in the art. Evidence (according to CVC) of the routine nature of these methods was that five other groups "quickly" performed CRISPR cleavage of eukaryotic DNA, as illustrated by this table:
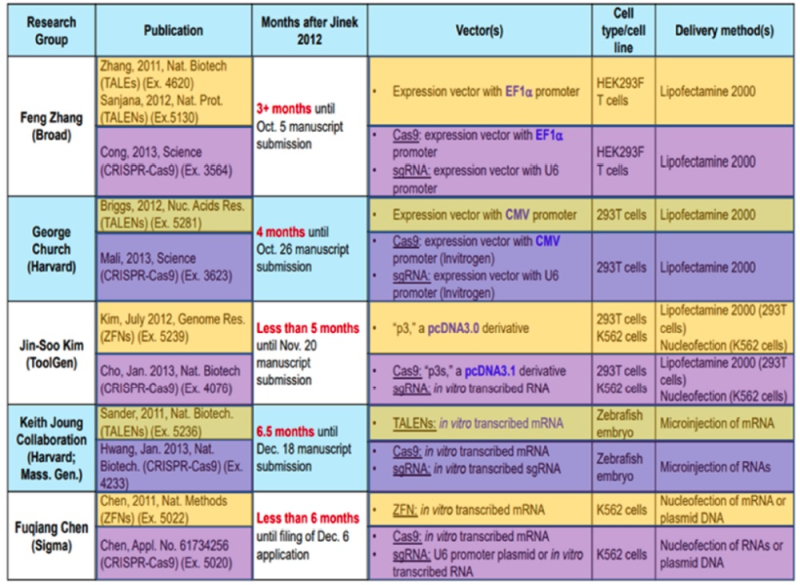
(where methods using prior art systems, such as ZFNs and TALENS are shown in orange and those using CRISPR are shown in purple). In this regard CVC argues that its own efforts, which formed the basis for the Board's determination that their conception was incomplete, took only four months of diligent effort.
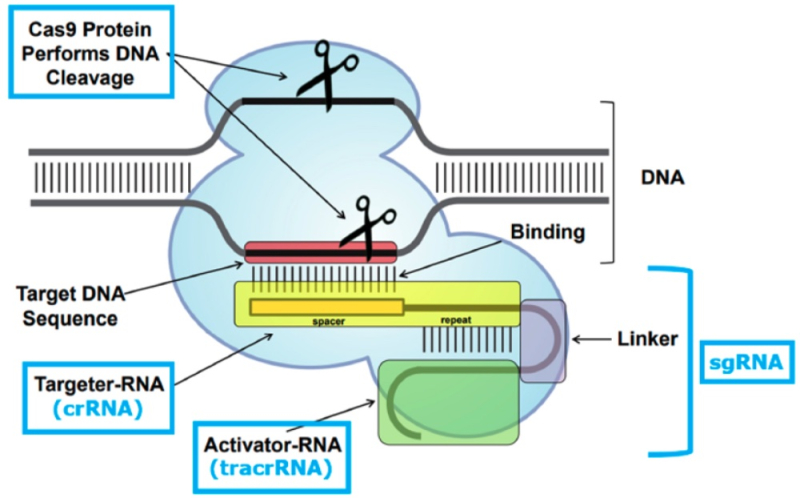
The recitation of the factual background recites that it was the CVC inventors who recognized the role the tracrRNA played in the CRISPR complex (in work showing DNA cleavage in vitro) and, most importantly, that the tracrRNA and target site-specific CRISPR RNA (crRNA) could be linked by an oligonucleotide linker to form sgRNA. Others, specifically the Broad group in CVCs retelling had been investigating whether there were "other factors" that needed to be included, such as RNase III, in so-called "preprocessing steps." CRISPR as conceived by CVC is illustrated in the brief by this graphic:
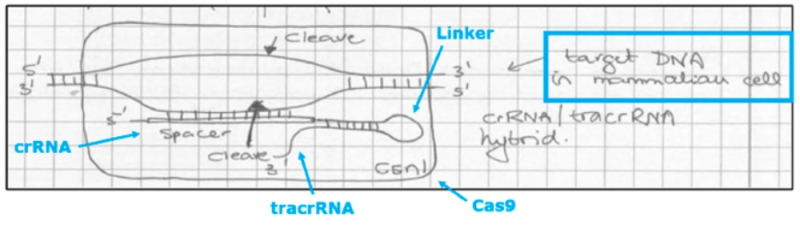
and the laboratory notebook from March 1, 2012 showing conception of sgRNA (termed "chimera A") is also provided:
Chimera A was later disclosed in CVC's scientific publication, A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity ("Jinek et al., 2012"):
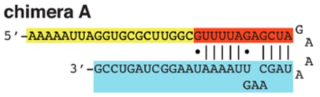
According to the brief, when used in their in vitro DNA cleavage system, embodiments of CRISPR comprising chimera A "cleaved the [DNA] target each time." This disclosure was included in CVC's P1 provisional application (No. 61/652,086, filed May 12, 2012), along with prophetic examples for producing or introducing sgRNA-containing CRISPR complexes into eukaryotic cells (including "fruit fly," "fish, amphibian, reptile, bird, mammal," and "human" cells) using prior art-recognized, "routine" techniques "(e.g., microinjection, electroporation, transfection, etc.)," according to CVC. Later-filed provisional applications (P2, filed October 19, 2012 and P3, filed January 28, 2013, the latter being the earliest priority date awarded to CVC by the Board) contained more details of their invention, the brief asserts.
According to the brief, the Broad group "had attempted—unsuccessfully—to use incomplete CRISPR-Cas9 systems to edit eukaryotic genes " because they did not understand "that mature tracrRNA was a necessary third component of the final DNA-cleavage complex" (emphasis in brief). Consequently, Broad "struggled with experiments using unprocessed RNAs and other extraneous elements, wondering what 'other factors need to be identified.'" Importantly, the brief asserts that it was only when Dr. Marraffini informed the Broad group of the CVC inventors disclosure of the sgRNA species that permitted Broad to reduce eukaryotic CRISPR to practice. Indeed, the brief identifies the Broad's asserted conception (June 26, 2012) to be the very day Dr. Marraffini disclosed sgRNA to them, illustrated by a copy of an e-mail:
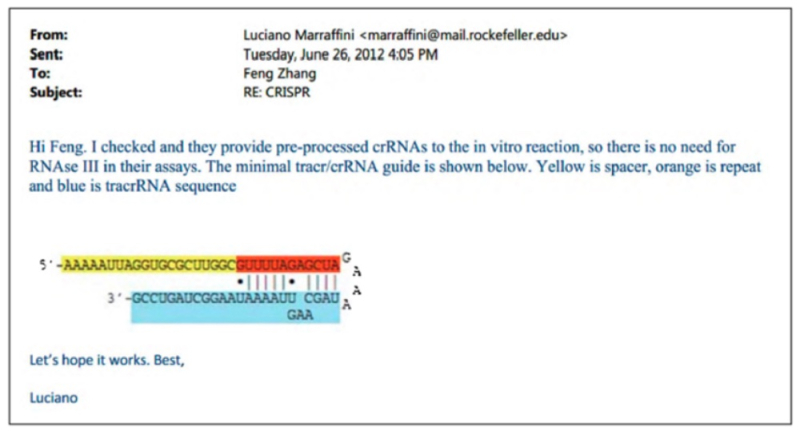
The brief also notes that "[d]espite years of litigation, including an earlier appeal to this Court [in Interference No. 106,048], Broad withheld that communication until discovery halfway through the proceedings below" (emphasis in brief). The brief goes on to describe the steps taken by Broad in reducing eukaryotic CRISPR to practice using the same chimera A sgRNA disclosed by CVC and communicated to Broad by Dr. Marraffini:
 The brief asserts that the CVC inventors had "conceived of and described every element of the invention before Broad's first alleged conception." The brief asserts further than the PTAB "never identified any inventive contribution Broad made" and awarded priority to Broad solely on the purported basis of first reduction to practice (emphasis in brief).
The brief asserts that the CVC inventors had "conceived of and described every element of the invention before Broad's first alleged conception." The brief asserts further than the PTAB "never identified any inventive contribution Broad made" and awarded priority to Broad solely on the purported basis of first reduction to practice (emphasis in brief).
With regard to their efforts to reduce sgRNA-comprising CRISPR in eukaryotic cells, the brief sets forth those attempts in ways that contradict or at least minimize the Board's basis for finding CVC's conception to be incomplete. For example, the brief emphasizes that the vectors used to produce sgRNA and Cas 9 protein were designed in May 2012 and ordered by June 2012. The brief also describes the efforts of a colleague to introduce preformed sgRNA-comprising CRISPR complexes into eukaryotic cells by microinjection into zebrafish. With regard to experiments using vectors to produce both sgRNA and Cas 9 protein in eukaryotic cells, the brief emphasizes that these experiments were initially performed by a graduate student (who CVC argues was not a person of ordinary skill in the art) who, after some preliminary positive results in CRISPR-mediated cleavage of eukaryotic DNA in vivo was unable to demonstrate homology-directed DNA repair in the cells. It was only after about three months of failure that the project was given to another graduate student who the brief asserts was able to reduce CRISPR-mediated cleavage to practice in eukaryotic cells "just weeks after joining Doudna's lab and only four months after CVC's first human-cell experiments." For the microinjection experiments, while positive results were obtained they did not demonstrate CRISPR cleavage with the efficiency obtained from in vitro experiments, and thus these results were never published (something the brief notes the PTAB interpreted as indicating a lack of success, despite this frequency, 1 in 30, being better than the frequency obtained in the Broad's initial experiments in mouse cells, 2 in 275).
The brief asserts several grounds of error by the PTAB. The first error is in refusing to apply an "objective" standard for conception, i.e., "whether CVC's invention was sufficiently complete that it was ready for skilled artisans to reduce it to practice without further invention." Instead, CVC argues that the PTAB required that the CVC inventors knew the invention would work, "disregard[ing] copious unrebutted evidence that artisans understood exactly how to reduce CVC's invention to practice using routine techniques," as illustrated by the evidence provided by synopsis in the table above. This was due to assertions by Broad that paralleled those made in the earlier '048 Interference with regard to the "theoretical hurdles to introducing the CRISPR-Cas9 complex into eukaryotic cells using expression vectors—RNA degradation, potential need for nuclear localization signals and codon optimization, and chromatin access," without the Board ever identifying which of those hurdles would prevent a skilled artisan from achieving CRISPR-mediate DNA cleavage in a eukaryotic cell according to the brief. The brief also asserts that the PTAB did not recognize or acknowledge that CVC "never changed its invention in any material way" nor determined whether the microinjection experiments represented an actual reduction to practice. Second, CVC argues that the PTAB did not identify "a single limitation of the count" that was independently provided by Broad. Third, CVC argues with regard to the PTAB's denial of their priority claim to their P1 and P2 provisional application that the Board used the wrong standard for written description: "[r]ather than assess whether CVC's patent disclosures were sufficient to allow skilled artisans to identify the invention, the PTAB demanded more: It required CVC's disclosures to persuade skeptical artisans the invention would overcome various imagined hurdles to reduction to practice in eukaryotic cells." Finally, CVC argued that the Board violated the Administrative Procedures Act because it "failed to engage in . . . reasoned decisionmaking" and its priority determination was thus arbitrary and capricious.
The brief supports these assertions of error on interference practice precedent. Regarding conception the brief argues that "CVC conceived of every limitation in the count before Broad's first alleged conception date of June 26, 2012." The Board's decision to the contrary, the brief argues, is based on "twin legal errors." The first is that it is hornbook law for over a century that "'conception' occurs once 'the inventor is ready to instruct the mechanic in relation to putting [the invention] in working form,'" citing Cameron & Everett v. Brick, 1871 C.D. 89, 90 (Comm'r Pat.), and that an idea is "sufficiently 'definite and permanent' if the inventors had 'both the idea of the invention's structure and possession of an operative method of making it,'" citing Amgen, Inc. v. Chugai Pharm. Co., 927 F.2d 1200, 1206 (Fed. Cir. 1991). Particularly relevant to the facts in this case, CVC argues that "[c]onception thus is complete when "[a]ll that remains to be accomplished . . . belongs to the department of construction, not invention," citing Mergenthaler v. Scudder, 11 App. D.C. 264, 276 (1897), that is, "when one of ordinary skill in the art could construct the apparatus without unduly extensive research or experimentation," citing Sewall v. Walters, 21 F.3d 411, 415 (Fed. Cir. 1994). Contrary to the standard in Sewall, that conception must provide the skilled artisan with a sufficient description that the invention could be reduced to practice, the brief argues that the Board ignored the evidence that five other laboratory groups, including Broad, achieved sgRNA-comprising CRISPR-mediated DNA cleavage in eukaryotic cells using CVC's disclosed invention. CVC argues that whatever challenges or delays were occasioned in their own efforts to reduce their invention to practice are "irrelevant" "when the evidence shows the invention is sufficiently firm, definite, and developed that skilled mechanics can do so," which CVC asserts the evidence shows was the case (emphasis in brief). The brief relies on Cameron for the principle that "[c]onception can be complete even if 'much patience and mechanical skill, and perhaps a long series of experiments,' are required to reduce the invention to practice." The brief then sets forth examples of instances where a patent was granted to the first to conceive even though an inventor did not, or could not, actually reduce the invention to practice. These cases include Dolbear v. American Bell Telephone Co., 126 U.S. 1 (1888)(Bell's invention of the telephone); the Wright Brothers invention of the airplane "nearly a year before their first successful flight and—despite an intervening series of discouraging tests—the patent was granted"; and the use of AZT to treat AIDS, which was conceived before demonstrating that the drug "actually worked," citing Burroughs Wellcome Co. v. Barr Labs., Inc., 40 F.3d 1223, 1228 (Fed. Cir. 1994).
The reason the PTAB "reached the wrong result" with regard to priority was because, CVC asserts, the Board "asked the wrong question," specifically by "insisting that inventors must know their invention would work for conception to be complete" because "[c]onception does not require even an 'expectation' the invention will work, much less knowledge it will," citing Burroughs Wellcome Co. and Univ. of Pittsburgh of Commonwealth Sys. of Higher Educ. v. Hedrick, 573 F.3d 1290, 1299 (Fed. Cir. 2009). The brief characterizes as "overwhelming" evidence that "CVC's invention was ready to be handed off to skilled artisans," as illustrated by the table from the brief set forth above. CVC argues that "[i]t is hard to imagine more powerful, objective, real-world evidence that conception was 'complete' . . . than the fact that so many actually did, so quickly after learning of CVC's invention." The brief also argues that the Board ignored the widespread recognition in the art, beginning with public disclosure of sgRNA-comprising CRISPR embodiments, that CRISPR was expected to be capable of cleaving DNA in eukaryotic cells (this evidence bolstered CVC argues by Broad reducing their invention to practice "within weeks" of receiving CVC's disclosure of chimera A).
Instead, CVC maintains, the Board was "doubly wrong" in focusing on purported subjective "uncertainty" by CVC inventors regarding their own attempts to reduce their invention to practice. First, the brief argues that "the 'existence of research or experimentation'—even 'a long series of experiments'—does not itself prove conception incomplete," citing Sewall and Cameron. What is determinative, CVC argues, is whether any experimentation required the exercise of "more than routine skill," citing Rey-Bellet v. Engelhardt, 493 F.2d 1380, 1387 (C.C.P.A. 1974), something CVC argues the Board never did. And the Board also never explained why the purported "months of failed experiments" were "too long" in view of their being performed by graduate students who were not persons of ordinary skill, CVC argues. Prior interference decisions (Dolbear, Hedrick) awarded priority based on evidence such as laboratory notebooks that "sufficiently described to those skilled in the art how to proceed." These rubrics were supported in CVC's brief by citation of other precedent, including Barba v. Brizzolara, 104 F.2d 198, 202 (C.C.P.A. 1939); Field v. Knowles, 183 F.2d 593, 603 (C.C.P.A. 1950); In re Tansel, 253 F.2d 241, 243-44 (C.C.P.A. 1958); Acromed Corp. v. Sofamor Danek Group, Inc., 253 F.3d 1371, 1380 (Fed. Cir. 2001); and Sewall. CVC's brief distinguishes the case, Alpert v. Slatin, 305 F.2d 891 (C.C.P.A. 1962), relied upon by the Board for incomplete conception being evidenced by failures in actual reduction to practice on the grounds that in that case the only evidence was the inventor's failure, whereas here multiple other groups including Broad were able to reduce sgRNA comprising CRISPR-mediated DNA cleavage in eukaryotic cells from CVC's disclosure of their invention.
CVC also contends that the Board was wrong in characterizing their reliance on the successful work of others to demonstrate their own complete conception as an attempt at nunc pro tunc conception, or that CVC was improperly attempting to use Broad's success to inure to their benefit, because there was nothing defective in their conception (which was used without revision or modification by Broad and others) and CVC did not fail to recognize its invention, as was the case in Hitzeman v. Rutter, 243 F.3d 1345, 1358 (Fed. Cir. 2001).
The Board, CVC argues, further erred in applying a "subjective expectation of success" standard, that "the inventors kn[e]w their invention would work" (emphasis in brief). This standard "defies" precedent, the brief maintains, citing City of Elizabeth v. Nicholson Pavement, 97 U.S. 126 (1877) (where the inventor was "not sure" the invention would work); Applegate v. Scherer, 332 F.2d 571, 573 (C.C.P.A. 1964); Dana-Farber Cancer Inst., Inc. v. Ono Pharm. Co., 964 F.3d 1365, 1372 (Fed. Cir. 2020); Hedrick; and Burroughs (although the Board stated that it did not "base [its] decision on a lack of reasonable expectation of success by the CVC inventors" the PTAB "contradicted that standard as quickly as it articulated it" according to the brief). And the brief in a footnote anticipates assertion of the "simultaneous conception and reduction to practice" standard of conception, which was earlier raised by Broad in some of their preliminary motions, by stating that PTAB's "failure to rely on it—and explain why it would apply—precludes its assertion on appeal" under In re Lee, 277 F.3d 1338, 1344-45 (Fed. Cir. 2002).
The brief then turns to what in some ways is the heart of the matter, regarding the question (the basis of all interferences) of who invented the invention of Count 1. According to CVC, the PTAB awarded priority to Broad based on first reduction to practice while identifying nothing the Broad inventors "actually invented," including no "adaptation" or "technical element" lacking in CVC's disclosure or that the skilled worker would have lacked. "Any test that awards inventorship to a party without identifying the inventive element he contributed cannot be right," CVC argues, because "[p]atent law rewards 'innovation,' not "'the work of a mechanic'" in reducing others' inventions to practice," citing Sinclair & Carroll Co. v. Interchemical Corp., 325 U.S. 327, 330 (1945). The brief argues that the PTAB concluded that "there must have been differences" between Broad's actual reduction to practice and CVC's based on the differences in when each party achieved actual reduction to practice. Because this is an "ipse dixit [that] is unexplained and inexplicable" the brief argues the Board never provided any reason or rationale the these "assumed" differences "reflected different inventive approaches, or [were] simply mechanical skill or luck." Nor did the Board acknowledge that Broad reduced the invention to practice using the same techniques [including chimera A] first disclosed by CVC. The brief answers its own rhetorical question -- why not? -- by asserting that the reason is that Broad derived its invention in toto from CVC, as evidenced by the activities of Dr. Marraffini (once again mentioning that these activities were kept undisclosed by Broad until "after a decade of patent prosecution and litigation"). CVC argues that "patents [are to] be awarded to an 'original inventor'—not a 'borrower or a copyist,'" citing 1 W. Robinson, The Law of Patents for Useful Inventions § 58 (1890) as well as the Constitution, and that someone who merely confirms another's conception using ordinary skill is not an "original" inventor, citing Applegate. CVC further argues that the PTAB "almost conceded" that, had CVC hired the Broad to reduce its invention to practice CVC and not Broad would be entitled to priority and states that the "result should not be different simply because [Broad], instead of being hired by CVC, took chimera A from CVC's still-unpublished manuscript and proved the invention works." "Patent rights to the invention of the century should not be awarded based on a footrace to implement it using routine techniques" according to CVC's brief.
CVC also argues that the Board contravened the standards and statutory requirements of the Administrative Procedures Act in arriving at its incorrect priority determination under the "reasoned decisionmaking" requirement and is thus arbitrary and capricious. Support for these allegations include the PTAB's failure to provide evidentiary support for its "must be differences" theory of CVC's alleged incomplete conception (the brief calls this "res ipsa reasoning") and the failure to identify the technical features purportedly lacking in CVC's attempts at actual reduction to practice. Citing Morall v. DEA, 412 F.3d 165, 178 (D.C. Cir. 2005), CVC argues that "[a]gency decisions must be grounded in evidence" and that the PTAB's priority determination lacked any such evidence, asserting:
Here, the PTAB did not identify any relevant difference between CVC's conception and Zhang's reduction to practice—because the record would not support any. Zhang used the same tools CVC had selected by June 2012 to reduce the invention to practice: expression vectors; standard promoters, including the U6 promoter; NLSs; and codon optimization. . . . The PTAB, moreover, did not explain how any of those techniques could make a difference: Broad conceded that none required more than ordinary skill. . . . Broad touted the "combination" as inventive, but never meaningfully disputed that CVC identified the same combination first [emphasis in brief].
Moreover, CVC argues that "[c]ourts cannot 'uphold agency action if' the agency decision 'fails to consider "significant and viable and obvious alternatives,"'" citing Dist. Hosp. Partners, L.P. v. Burwell, 786 F.3d 46, 59 (D.C. Cir. 2015), which "precisely describes the PTAB's decision here" according to CVC. After all, Broad's purported success rate (0.75%) at sgRNA-comprising CRISPR-mediated DNA cleavage in mammalian cells was not demonstrably better than success rates by CVC scientists that they deemed inconclusive (e.g., one transformed zebrafish embryo in 30, or ~3%) CVC argues. In addition, CVC argues that "the line between CVC's supposed failures and its successes was not drawn by a change in techniques. It followed a change in graduate students" and could be the result of "random chance . . . human error, equipment quality, and measurement failures." And many of the first graduate student's "failures" were the result of attempts to reduce CRISPR embodiments (HDR) that were outside the scope of the count (which was limited to CRISPR-mediated DNA cleavage).
CVC also argued that the Board "ignored rafts of evidence contrary to its position" (or conclusions). The brief cites Princeton Vanguard, LLC v. Frito-Lay N. Am., Inc., 786 F.3d 960, 970 (Fed. Cir. 2015), for the proposition that under the APA "the PTAB must address the evidence for and against the results it reached," and then recites 4 species of such evidence they argue the Board did not consider. These included contemporary opinion by "CRISPR luminaries" regarding how "straightforward" application of CRISPR-mediated DNA cleavage would be in eukaryotic cells; that five other labs were able to reduce CRISPR cleavage methods within the scope of the interference count "shortly" after CVC announced its conception of sgRNA-comprising CRISPR embodiments; that CVC (and everyone else) ultimately achieved actual reduction to practice of the invention CVC had conceived with no changes; and that CVC was in fact subjectively confident that its invention "would work in eukaryotic cells," contrary to the meme of doubt successfully argued to the Board by Broad. In this regard CVC brought up the (relatively) short time (four months) between conception and undisputed actual reduction to practice and that the Board "never explained why a four-month [delay in] reduction to practice amounts to 'perplexing' difficulties 'every step of the way,'" contrasting this time with difficulties encountered by the Wright Brothers and Bell for reducing their inventions to practice. "Nowhere did the PTAB explain why the inventors awarded the Nobel Prize for probably the most stunning advance of the century should be disqualified because their graduate students initially stumbled but ultimately succeeded in just four months. That lack of reasoned explanation is arbitrary and unsustainable," CVC argued. And the Board's treatment of the microinjection experiments "fares worse still, CVC argues because "the PTAB simply refused to engage with evidence that there is 'something different about microinjection' that 'negates' the purported hurdles that (according to the PTAB) might prevent implementing the count with vectors, and this failure is a violation of the APA, citing Provisur Techs., Inc. v. Weber, Inc., — F.4th —, 2022 WL 4474941, at *4-5 (Fed. Cir. Sept. 27, 2022). CVC characterizes the Board's analysis of these experiments as comprising "bare assertion without 'substantial evidence,' or any evidence, behind it" because it did not dispute that "microinjecting a pre-formed complex into rapidly dividing cells like zebrafish embryos obviates most, if not all, of" the purported obstacles in using vectors to express functional sgRNA and Cas9 in eukaryotic cells. The Board's decision with regard to CVC's reduction to practice without considering these differences was thus arbitrary and capricious, according to CVC. And the brief raises similar objections to the Board's application of the written description requirement to the microinjection experiments based on purported obstacles arising from the vector-mediated experiments (because microinjection of preformed sgRNA-comprising CRISPR complexes obviated such obstacles).
The brief concludes with CVC's arguments regarding the Board's application of the written description requirement to the question of whether CVC was entitled to the priority dates of its P1 or P2 provisional applications (which if granted would have make CVC the Senior Party, with the advantages regarding burdens of proofs and presumptions attendant thereto). Citing Alcon Rsch. Ltd. v. Barr Labs., Inc., 745 F.3d 1180, 1190 (Fed. Cir. 2014), Crown Packaging Tech., Inc. v. Ball Metal Beverage Container Corp., 635 F.3d 1373, 1380-81 (Fed. Cir. 2011), and Ariad Pharms., Inc. v. Eli Lilly & Co., 598 F.3d 1336, 1351 (Fed. Cir. 2010) (en banc), CVC argues that the Board used an incorrect standard; in an interference the proper standard entails providing a description of even "one embodiment within the scope of the court" to be enough to constitute a constructive reduction practice, citing Falkner v. Inglis, 448 F.3d 1357, 1362 (Fed. Cir. 2006). CVC argues that its P1 provisional provides such a description of expressing sgRNA-comprising CRISPR-mediate DNA cleavage on a eukaryotic cell, i.e., how to make and use the invention. The PTAB's error was in requiring that P1 do more than this, needing to "convince skilled artisans that the invention would work" according to CVC. (While enunciating the extent of the disclosure relating to sgRNA-comprising CRISPR-mediated DNA cleavage in eukaryotic cells in P1 what remains unmentioned in the brief is that this disclosure is prophetic with regard to in vivo embodiments.) The brief cites Evans v. Eaton, 20 U.S. (7 Wheat.) 356, 434 (1822), for the principle that P1 discloses what CVC claims as its invention, and Crown Packaging to support CVC's conception of this invention. The brief argues that P1 discloses the "scientific principles underlying [the] invention" and discloses how to use CRISPR-Cas9 in eukaryotic cells using the same "well-known techniques" used in prior art TALENS and ZFN methods, including microinjection and use of expression vectors as well as including nuclear location sequences and codon optimization for Cas9 protein components. In the face of this disclosure CVC argues that the Board erred by "improperly engraft[ing] a burden-to-convince requirement onto written description." The proper standard is disclosure sufficient for the skilled worker to "recognize that what was claimed corresponds to what was described" and "not about whether the patentee has proven to the skilled reader that the invention works," citing Alcon. The Board erred, according to CVC, because it required evidence in the P1 disclosure that what was described "worked," i.e., a disclosure of actual rather than constructive reduction to practice. CVC argues that the law does not require such a showing (Alcon) nor disclosure of "examples," "data" or "prior experimental work" (Ariad). And delay by an inventor in achieving actual reduction to practice does not negate a showing of possession of an adequately disclosed invention the brief states, citing BASF Plant Sci., LP v. Commonwealth Sci. & Indus. Rsch. Org., 28 F.4th 1247, 1267 (Fed. Cir. 2022). CVC's brief enumerates the evidence the Board improperly required, including "data from eukaryotic experiments"; how "all possible difficulties" were to be overcome; disclosure of "specific instructions or conditions necessary for CRISPR-Cas9 activity in a eukaryotic cell"; and "[c]onvincing skeptics they should not 'doubt' the in vitro experiment results." Precedent holds that a lack of adequate written description involves a failure to disclose not a failure to persuade, CVC argues, citing Ariad and Biogen Int'l GMBH v. Mylan Pharms. Inc., 18 F.4th 1333, 1343-44 (Fed. Cir. 2021), and the Board's requirement for persuasion in the P1 priority document was thus error. And the nature and root of this error is illustrated, according to CVC, by the Board's acknowledgement by awarding priority to the P3 provisional application, which differed from P1 and P2 only because it included a working example of sgRNA-comprising CRISPR-mediated DNA cleavage in a eukaryotic cell. CVC summarizes this argument by stating:
No inventor can anticipate and preempt with instructions every single litigation-inspired hypothetical problem that can be conjured. Lawyers can always imagine 1,001 reasons an invention might not work. Paid experts can invent still more. "'[W]hen the question is whether a thing can be done or not, it is always easy to find persons ready to show how not to do it.'" Dolbear, 126 U.S. at 536. For groundbreaking inventions like this, it is easier still to hypothesize why they might fail. But written description is description of the invention, not proof it works. "Possession" means possession of the idea, not construction of a working example. The patent system does not punish inventors of breathtaking innovations by saddling them with the burden of convincing putative skeptics their invention will work.
The brief concludes by asking the Federal Circuit to vacate the PTAB's judgment and findings and to reverse or remand, presumably for consideration under the proper interpretation of the law as set forth in CVC's brief.