 In the first of a pair of decisions issued last Friday, Ferring B.V. v. Watson Laboratories, Inc., the Federal Circuit affirmed a finding by the District Court that a generic company could moot ANDA litigation by amending its application to exclude practice of an infringing article.
In the first of a pair of decisions issued last Friday, Ferring B.V. v. Watson Laboratories, Inc., the Federal Circuit affirmed a finding by the District Court that a generic company could moot ANDA litigation by amending its application to exclude practice of an infringing article.
The case involved a generic form of Lysteda® (trans-4-(aminomethyl)cyclohexanecarboxylic acid, also called tranexemic acid), used to treat heavy menstrual bleeding. The drug was known in the prior art, but was also known to be associated with dosage-dependent gastrointestinal side effects. The Orange Book-listed patents-in-suit, U.S. Patent Nos. 7,947,739, 8,022,106, and 8,273,795, were directed to "modified release" formulations; claim 1 of the '106 patent is representative (according to the Court):
1. A tranexamic acid oral dosage form comprising:
tranexamic acid or a pharmaceutically acceptable salt thereof; and
a modified release material . . . ;
wherein the modified release material is present in the formulation in an amount from about 10% to about 35% by weight [i.e., "wt%"] of the formulation;
wherein said dosage form provides an in-vitro dissolution release rate of the tranexamic acid or pharmaceutically acceptable salt thereof, when measured by a USP 27 Apparatus Type II Paddle Method @ 50 RPM in 900 ml water at 37±0.5°C., of less than about 40% tranexamic acid or pharmaceutically acceptable salt thereof released at about 15 minutes, less than about 70% by weight tranexamic acid or pharmaceutically acceptable salt thereof released at about 45 minutes and not less than about 50% by weight of said tranexamic acid or pharmaceutically acceptable salt thereof released by about 90 minutes; and
wherein each tranexamic acid oral dosage form provides a dose of about 650 mg tranexamic acid.
As discussed in the opinion, this formulation was intended to "match" the release rate of the drug with the drug uptake rate in vivo, thus reducing the adverse gastrointestinal side effects.
The opinion set forth the following table, showing the dissolution characteristics of the various formulations and the presence of each limitation in the broadest independent claim in each of the patents in suit; the opinion states that these characteristics are dispositive with regard to whether defendant Apotex's generic formulation infringes:
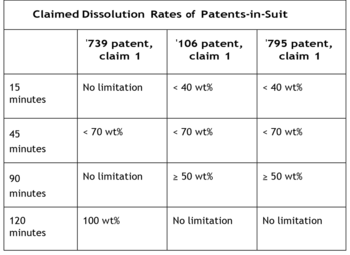
With regard to these characteristics, Apotex's original ANDA filing specified formulations wherein at least 80% of tranexamic acid would dissolve in 60 min.
The District Court construed a single term in the claims, concerning the meaning of the word "about" as used in the claim limitation "less than about [%] by weight" (emphasis in opinion). Both parties argued that the term "demarcate[d] particular numerical ranges," with Ferring contending that the word meant "within 10% [i.e., 'about 70%' means 63-77%]" and Apotex maintaining that it meant "within 5% [i.e., 'about 70% means 66.5-73.5%]. The District Court disagreed with both parties, construing the word to mean "approximately."
The experimental data adduced at trial from Apotex's "biobatches" showed that the generic formulation did not fall within either percentage range, but the District Court decided that that because Apotex's (original) ANDA did not specify the dissolution rate "the ANDA permitted Apotex to sell an infringing product" and "permitted [Apotex] to violate the patent," relying on its interpretation of Sunovion Pharmaceuticals, Inc. v. Teva Pharmaceuticals, Inc., 731 F.3d 1271 (Fed. Cir. 2013). In view of this determination, Apotex agreed that it would amend its ANDA to specify that its formulations would have a dissolution profile of "not less than 75 percent by weight of the tranexamic acid was released at 45 minutes." Apotex further made representations to Ferring, the District Court, and the FDA that it would not market a generic version of the branded drug having a different dissolution profile without informing Ferring, the District Court, and the FDA of the change. The District Court then dismissed the lawsuit based on these changes and representations.
The Federal Circuit affirmed, in an opinion by Judge Dyk joined by Judges Lourie and Reyna. The opinion reviewed the judgment of non-infringement under both the original and amended ANDA. With regard to the original ANDA, the Court held that the District Court erred in determining that the failure to specify a dissolution profile in the first ANDA and had misinterpreted the Court's Sunovion decision. Specifically, the panel disagreed with Ferring and the District Court that the Federal Circuit's Sunovion opinion was controlling, because the facts here are not the same as the facts in Sunovion. In that case, the Court stated, "the ANDA specified an infringing product," and that the ANDA applicant could not avoid infringement by "certifying that it would not infringe, overriding the language of its own ANDA." Here, the ANDA was silent regarding the characteristics that would establish infringement and thus did not specify an infringing product. Under these circumstances, the panel identified the correct precedent that governs to be Glaxo, Inc. v. Novopharm, Ltd., 110 F.3d 1562, 1570 (Fed. Cir.1997). The panels reasoning was based on who bears the burden of establishing infringement, the Federal Circuit disagreeing with Ferring, who argued that Apotex was bound to show that its product does not infringe (because whether or not it infringed was not specified in the ANDA). On the contrary, the Court ruled that the burden is on Ferring. And here, the evidence was that the Apotex product (from "bio-batch data") showed the Apotex product does not infringe. While this parsing of the evidentiary burdens is consistent with (but not dependent on) the Supreme Court's recent decision in Medtronic v. Mirowski, these circumstances may impose upon Ferring an on-going duty to monitor Apotex's marketed product that is an added burden.
With regard to infringement in view of the later amendments to Apotex's ANDA, the Federal Circuit reviewed the District Court's claim construction regarding the meaning of the word "about." If Ferring's construction is correct then the amended ANDA could infringe (because the "less than 75%" limitation falls within the +/- 10% interpretation espoused by Ferring (i.e., 70% is within the 63-77% range), while if Apotex's construction is adopted then its generic formulation would not (75% is not within the range of 66.5-73.5%). The Federal Circuit rejected the factual grounds (the U.S. Phamracopeia) asserted by Ferring for construing "about" to implicate its 10% variance in the specified about. Finding that the meaning of the word "about" was not defined in the specification, the Federal Circuit held that the District Court correctly construed the word "about" according to its ordinary and customary meaning to be "approximately," citing Merck & Co., Inc. v. Teva Pharms. USA, Inc., 395 F.3d 1364, 1369–70 (Fed. Cir. 2005) for that proposition. Using this construction, the Court further found that Ferring did not bear its burden of showing infringement ("Ferring, the party with the burden of proof on infringement, [produced no] evidence that the 2014 ANDA infringed by proposing a 75 percent by weight dissolution rate, under the district court's claim construction").
Finally, the opinion addressed Ferring's contention that under § 271(e)(4)(A), the District Court was compelled to order a change in the Apotex ANDA effective date, based on a finding of infringement under the terms of the ANDA as originally filed. The Federal Circuit rejected this proposition, finding that a district court should evaluate infringement by filing an ANDA on the basis of the originally filed ANDA and any amendments or other changes submitted to the FDA, citing Bayer AG v. Elan Pharmaceutical Research Corp., 212 F.3d 1241 (Fed. Cir. 2000). While the opinion recognizes that whether an amendment to an ANDA is considered is within the sound discretion of the district court, "guided by principles of fairness and prejudice to the patent-holder," the statute does not require resetting the ANDA filing date automatically with the filing of an amendment. Here, the District Court's refusal to reset was not an abuse of discretion according to the Court.
The Court also ordered costs to Apotex, suggesting that the panel did not believe that this was a sufficiently close case that justified Ferring's appeal.
Ferring B.V. v. Watson Laboratories, Inc. (I) (Fed. Cir. 2014)
Panel: Circuit Judges Lourie, Dyk, and Reuna
Opinion by Circuit Judge Dyk