The Federal Circuit applied the constitutional principle under Article III that there must be a case or controversy for a federal court to enter judgment (in this case, of invalidity) in ANDA litigation that can be vitiated by a statutory disclaimer of patent claims prior to judgment. The Court also applied principles of chemical obviousness and its "lead compound" analysis to affirm the District Court's determination that Defendants had not shown that claims in a related patent-in-suit were obvious.
The case arose in ANDA litigation involving Sanofi's Jevtana® (cabazitaxel), having the structure:
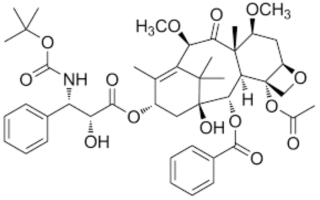
where the methoxy groups at the C7 and C10 positions distinguished cabazitaxel from prior art docetaxel, which had hydroxyl groups at these positions. At trial, Sanofi initially asserted claims 7, 11, 14-16, 21, 26, and 30 of Orange Book-listed U.S. Patent No. 8,972,592, which claims methods for treating drug-resistant prostate cancer with cabazitaxel, and U.S. Patent No. 5,847,170, which claims the compound itself as well as pharmaceutical compositions thereof:
Claim 1:
1. 4α-Acetoxy-2α-benzoyloxy-5β,20-epoxy-1β-hy-droxy-7β,10β-dimethoxy-9-oxo-11-taxen-13α-yl(2R,3S)-3-tert-butoxycarbonylamino-2-hydroxy-3-phenylpropionate.
Claim 2:
2. A pharmaceutical composition comprising at least the product according to claim 1 in combination with one or more pharmaceutically acceptable diluents or adjuvants and optionally one or more compatible and pharmacologically active compounds.
The opinion set forth the developmental history of cabazitaxel:
Cabazitaxel was the product of a multi-year research program aimed at identifying taxane analogs with better activity than docetaxel in resistant tumors. By making substitutions at multiple positions on docetaxel with various functional groups, Sanofi scientists synthesized several hundred compounds and tested their activities. Of this group, cabazitaxel was one of two compounds that entered into human studies. It obtained FDA approval in 2010.
While ANDA litigation was proceeding, the Patent Trial and Appeal Board (PTAB) invalidated all asserted claims of the '592 patent for obviousness. Facing denial of its motion to amend in the IPR, Sanofi filed a statutory disclaimer of claims 7, 11, 14-16, and 26. (In a footnote, the opinion notes that the Federal Circuit thereafter vacated the Board's decision and remanded for consideration regarding Sanofi's motion to amend during the IPR, which remains pending.) Despite this, the District Court entered judgment against all claims of the '592 patent that had been raised at trial for being invalid for obviousness; separately the Court entered judgment that Defendants had failed to show claims 1 and 2 of the '170 patent were invalid for obviousness.
The Federal Circuit vacated the District Court's decision regarding disclaimed claims 7, 11, 14-16, and 26 of the '592 patent because no case or controversy existed at the time the Court issued its opinion, and affirmed the District Court with regard to the '170 patent, in an opinion by Judge Lourie joined by Judges Moore and Taranto. On appeal, Defendants argued that the possibility that Sanofi would be able to amend the claims in IPR maintains a case or controversy with regard to "future" issue or claim preclusion defenses in possible future litigation between the parties. The Federal Circuit agreed with Sanofi that the statutory disclaimer removed the "case or controversy" requirement under Article III of the Constitution. This requirement, the Court asserts, must be "'real and substantial' and 'admi[t] of specific relief through a decree of a conclusive character, as distinguished from an opinion advising what the law would be upon a hypothetical state of facts,'" citing MedImmune, Inc. v. Genentech, Inc., 549 U.S. 118, 127 (2007) (alteration in original) (quoting Aetna Life Ins. Co. v. Haworth, 300 U.S. 227, 240–41 (1937)). The requirement is "highly similar" to the standing requirement, which is that "a plaintiff must 'present an injury that is concrete, particularized, and actual or imminent; fairly traceable to the defendant's challenged behavior; and likely to be redressed by a favorable ruling,'" citing Dep't of Commerce v. New York, 139 S. Ct. 2551, 2565 (2019) (quoting Davis v. Fed. Election Comm'n, 554 U.S. 724, 733 (2008)). Finally, the opinion states that the "actual controversy must be extant at all stages of review, not merely at the time the complaint is filed," citing Steffel v. Thompson, 415 U.S. 452, 459 n.10 (1974) (emphasis added). Applying these principles to the facts, the Court had little difficulty arriving at the conclusion that, at the time the District Court entered its judgment there was no controversy with regard to these claims, which Sanofi had disclaimed (which left the '592 patent "as though the disclaimed claim(s) had 'never existed,'" citing Genetics Inst., LLC v. Novartis Vaccines & Diagnostics, Inc., 655 F.3d 1291, 1299 (Fed. Cir. 2011) (quoting Vectra Fitness, Inc. v. TNWK Corp., 162 F.3d 1379, 1383 (Fed. Cir. 1998)).
The panel rejected Defendants' allegation that they would lose the possibility of the issue preclusion defense "should Sanofi obtain amended claims and assert them against Defendants." This is not sufficient to satisfy the case or controversy requirement, according to the opinion, first because the relevance of the disclaimed claims to a future issue preclusion defense was speculative, and second, the Defendants failed to establish that the District Court judgment pertaining to the disclaimed claims "is material to a possible future suit." And the panel refused Defendants' invitation to provide an advisory opinion on "the claim preclusion arguments that they intend to make . . . should Sanofi secure amended claims at the Board and then assert them against Defendants."
Turning to the District Court's decision that Defendants had not shown by clear and convincing evidence that claims 1 and 2 of the '170 patent were invalid for obviousness, the panel relied on the Court's decision in Takeda Chem. Indus., Ltd. v. Alphapharm Pty., Ltd., that a challenger must "identify some reason that would have led a chemist to modify a known compound in a particular manner to establish prima facie obviousness of a new claimed compound." 492 F.3d 1350, 1357 (Fed. Cir. 2007). The opinion reviewed the District Court's "extensive [factual] findings" based on the testimony of "seven witnesses and seventeen prior art references" in arriving at its conclusion that the District Court had not erred. This analysis centered on the question of the motivation and rationale of the skilled worker to modify prior art docetaxel by simultaneously replacing hydroxyl groups with methoxy compounds at positions C7 and C10. Defendants argued that this modification would be motivated to increase the lipophilicity of docetaxel to interfere with its binding by P-glycoprotein (Pgp), a plasma membrane-associated protein pump that rendered cells resistant to cytotoxic drugs (like docetaxel) by extruding these compounds from the cell. The panel credited the District Court's determination that the prior art cited in support of increased lipophilicity as a way to decrease Pgp extrusion did not disclose taxanes or show any relationship between lipophilicity and Pgp extrusion for taxanes. Concerning Defendants' assertion of prior art related to possible substitution positions in the canonical taxane structure, the panel agreed with the District Court's characterization that Defendant had cherry-picked the data in the cited references to reach the pattern of substituents exhibited by cabazitaxel and thus rejected them. Secondary considerations (commercial success, failure of others) also supported the District Court's decision that Defendants had not established obviousness of claims 1 and 2 of the '170 patent by clear and convincing evidence.
The Federal Circuit also rejected Defendants' argument in their declaratory judgment counterclaims that "a skilled artisan would have: (1) been motivated to modify docetaxel to reduce Pgp-related drug resistance; (2) knew that this could be accomplished by increasing lipophilicity of the C7 and C10 positions; and (3) determined that methoxy substitutions were the 'smallest, most conservative' modification to achieve that goal" as the product of hindsight. The panel reviewed the District Court's assessment of the asserted prior art, the deficiencies of this art and Defendants' arguments and reached the conclusion that these arguments were the product of hindsight.
Sanofi-Aventis U.S., LLC v. Fresenius Kabi USA, LLC (Fed. Cir. 2019)
Panel: Circuit Judges Lourie, Moore, and Taranto
Opinion by Circuit Judge Lourie