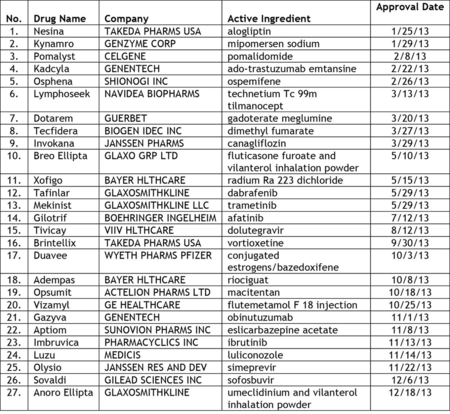
 Last month, the U.S. Food and Drug Administration (FDA) announced that the agency approved 27 new drugs and biological products in 2013. According to the FDA release, the 27 new molecular entities (NMEs) that were approved last year include:
Last month, the U.S. Food and Drug Administration (FDA) announced that the agency approved 27 new drugs and biological products in 2013. According to the FDA release, the 27 new molecular entities (NMEs) that were approved last year include:
 The number of new drugs approved in 2013 fell by almost a third from the 39 new drugs approved in 2012 ("New Molecular Entity Approvals for 2012"), and was three less than the 30 new drugs approved in 2011 ("New Molecular Entity Approvals for 2011").
The number of new drugs approved in 2013 fell by almost a third from the 39 new drugs approved in 2012 ("New Molecular Entity Approvals for 2012"), and was three less than the 30 new drugs approved in 2011 ("New Molecular Entity Approvals for 2011").
According to a Reuters report, the FDA attributed the drop in approvals to a drop in the number of applications filed, while noting that the 27 approvals were just under the 5-year average of 28.