Last week, the Federal Circuit confirmed that Idenix Pharmaceuticals will not be the proud recipient of what was previously regarded as the largest damages award ever recorded in a U.S. patent case. In fact, the majority’s opinion in Idenix Pharmaceuticals LLC v. Gilead Sciences Inc. not only affirmed the district court’s grant of judgment as a matter of law (JMOL) against Idenix and finding that the asserted claims of U.S. Patent No. 7,608,597 were invalid for lack of enablement under 35 U.S.C. § 112, it added insult to injury when it also held that the claims were invalid for lacking written description.
Background
Idenix (acquired by Merck & Co. in 2014) owns the ʼ597 patent, which includes claims directed to a method of treating hepatitis C virus. In an effort to thwart the launch of Gilead’s first drug for the treatment of chronic hepatitis C, Sovaldi®, which was then awaiting approval by the FDA, Idenix sued its rival for patent infringement in the District of Massachusetts in 2013. While the suit, which was ultimately transferred to the District of Delaware, was percolating, both Sovaldi® and a second drug Harvoni® were approved by the FDA for market. The drugs were quickly considered blockbuster drugs for their ability to cure hepatitis C in a large percentage of patients. However, there was also major controversy over the list price of the drugs—$84,000 for a 12-week regimen of Sovaldi® and $94,500 for a 12-week regimen of Harvoni®. Not surprisingly, the high price tag and cure rate resulted in huge revenue for Gilead. In fact, in 2015, Gilead reported it earned over $20 billion on the two drugs. Suffice it to say, there was a lot at stake in the dispute for both sides.
Claim 1 is illustrative of the asserted claims:
- A method for the treatment of a hepatitis C virus infection, comprising administering an effective amount of a purine or pyrimidine β-D-2′-methyl-ribofuranosyl nucleoside or a phosphate thereof, or a pharmaceutically acceptable salt or ester thereof.
After Idenix argued that the key to the claimed invention was the use of nucleosides having a methyl group (CH3) at the 2’ up position, the district court construed the structural limitation “β-D-2′-methyl-ribofuranosyl nucleoside” to require “a methyl group in the 2’ up position and non-hydrogen substituents at the 2’ down and 3’ down positions.” The 2’ up position is 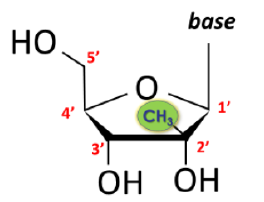 shown in the green circle in the structure to the left. The district court also construed the claims to use a β-D-2′-methyl-ribofuranosyl nucleoside that is effective for the treatment of hepatitis C.
shown in the green circle in the structure to the left. The district court also construed the claims to use a β-D-2′-methyl-ribofuranosyl nucleoside that is effective for the treatment of hepatitis C.
Based on the claim construction, Gilead ultimately conceded infringement before trial. But, it argued that under the claim construction, the asserted claims of the ʼ597 patent were invalid for both lack of enablement and written description under U.S.C. § 112(a). Gilead’s rationale was that there are billions of potential nucleosides with a methyl group in the 2’ up position and that a person of ordinary skill in the art would not know, without undue experimentation, which of these nucleosides would be effective for treating hepatitis C. So, while the jury was relieved of considering infringement, it was tasked with deciding whether the claims were valid under § 112 and if so, what damages were to be paid by Gilead.
In late 2016, the jury sided with Idenix when it upheld the validity of the asserted claims of the ʼ597 patent and awarded Idenix over $2 billion (based on a 10% royalty rate from the sales of both drugs). Gilead pleaded with the district court in post-trial motions to overturn the verdict and also hold that the claims were invalid for failing to satisfy the written description requirement under § 112(a). While the district court granted Gilead’s JMOL on enablement, it denied the motion with respect to written description. Idenix appealed.
The Majority’s Opinion
The Federal Circuit affirmed the lower court’s decision on enablement but reversed the denial of JMOL on written description. In explaining its affirmation of the lack of enablement finding, the majority weighed each of the Wands factors that the jury had before it:
(1) the quantity of experimentation necessary;
(2) how routine any necessary experimentation is in the relevant field;
(3) whether the patent discloses specific working examples of the claimed invention;
(4) the amount of guidance presented in the patent;
(5) the nature and predictability of the field;
(6) the level of ordinary skill; and
(7) the scope of the claimed invention.
In re Wands, 858 F.2d 731, 737 (Fed. Cir. 1988). The parties themselves had agreed on the high level of ordinary skill and experts from both sides concurred as to the unpredictability of the field. As such, both of these factors were found to weigh in favor of non-enablement. As to the quantity of experimentation necessary, the court agreed with the lower court’s finding that excessive experimentation would have been required to determine which 2’-methyl nucleosides would be effective for treating hepatitis C virus. In addition, the majority explained that the working examples and guidance in the ʼ579 patent merely provided a starting point for further research, but did not go so far as to allow a skilled artisan to know which 2’-methyl nucleosides are or are not effective against hepatitis C virus. The final factor—scope (overbreadth) of the claims—also weighed in favor of non-enablement. Thus, weighing each of the factors, the majority concluded as a matter of law that the asserted claims of the ʼ597 patent were invalid for lack of enablement. In further supporting its decision, the majority likened the facts of this case to those in Wyeth and Cordis Corp. v. Abbott Laboratories, where the claims encompassed millions of compounds, while only a much smaller subset of those compounds would have actually had the functional effect required by the claims. As here, the court in Wyeth found that having to synthesize and screen each potential compound for effectiveness was undue experimentation.
On the written description issue, the majority held that the ʼ579 patent did not adequately demonstrate that the inventors possessed the full scope of the claimed invention, and certainly not if the scope covered Gilead’s Sovaldi® and Harvoni® drugs. The majority’s opinion explained that, even though “a nucleotide-by-nucleotide recitation of the entire genus” is not required to find adequate written description, the absence of explicit disclosure in the ʼ597 patent of the particular species found in Gilead’s drugs (2’ methyl up/2’ fluoro down nucleoside), and further absence in the ʼ597 patent of a sufficient number of species to define a genus that includes the species found in Gilead’s drugs, or structure/function relationships that would necessarily include the species found in Gilead’s drugs make it clear that the ʼ597 patent fails to satisfy the written description requirement. Interestingly, even though its lack of enablement affirmation was partially based on the rationale that the ʼ579 patent was insufficient with its working examples and guidance, the court supported its finding of failure to the written description requirement with reference to “tens or hundreds of thousands of possible nucleosides, substituent-by-substituent, with dozens of distinct stereochemical structures…”
In Judge Newman’s dissent, she sets forth her view that there is no lack of enablement or written description because the claims are limited by what is set forth in the specification, such that interpreting claim scope necessarily restricts the scope to that disclosure. She also concludes that even though Gilead stipulated to infringement, the proper outcome of the case should be that the asserted claims are valid based on the scope supported by the specification, but not infringed since the species of 2’-methyl nucleosides used in Gilead’s accused drugs were not included in the scope of the asserted claims. The majority responded that Judge Newman’s preferred outcome on appeal would disregard binding claim construction, ignore the stipulation of infringement by Gilead, and analyze a case not before the court on appeal. In any event, the majority’s conclusion and the dissenting opinion result in the same outcome for Gilead, i.e., no damages. The major difference is that the majority’s opinion has eviscerated yet another pharmaceutical patent, whereas the dissent’s view would have preserved the patent while clearing Gilead of infringement.