The Federal Circuit reviewed the latest decision from the Patent Trial and Appeal Board (PTAB) in an inter partes review that claims 3-6 and 10 of U.S. Patent No. 6,548,019 are obvious, in Rembrandt Diagnostics LP v. Alere, Inc.; prior proceedings were reported at Alere, Inc. v. Rembrandt Diagnostics, LP, 791 F. App'x 173 (Fed. Cir. 2019), and Rembrandt Diagnostics, LP v. Alere, Inc., 809 F. App'x 903 (Fed. Cir. 2020).
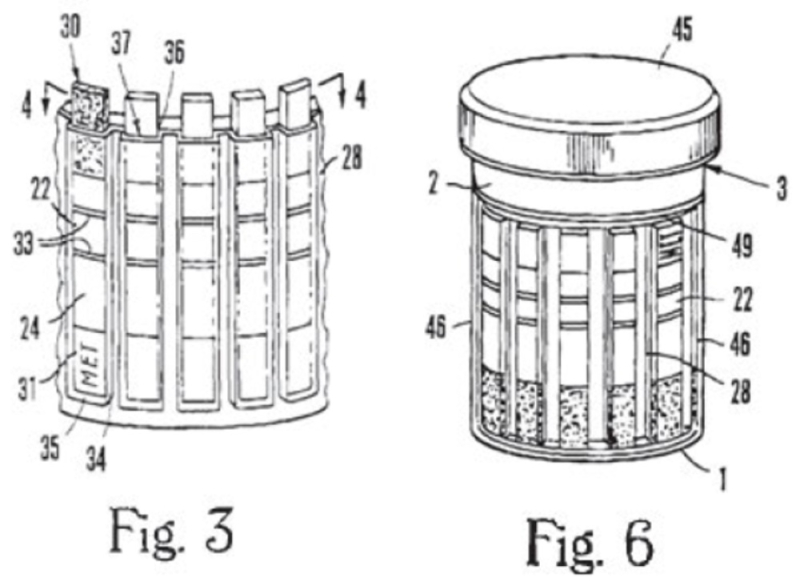
The claims are directed to assay devices for testing biological fluids, as illustrated in Figures 3 and 6 and explained in the opinion as follows:
The test assay device receives a fluid sample "introduced directly to the sample loading zone" (30) of one or more assay test strips (22) . . . the assay test strips (22) may each be encapsulated within separate "flow control channel[s]" (34) in one embodiment. . . . Figure 6 illustrates that to assemble the full testing assay device, the test strip assembly (22, 28, 34) from Figure 3 may be combined with a holder (40) and placed inside a fluid sample container (i.e., a cup) (2) that has a cap (45) to fit over the opening (3) of the container. . . . Figure 6 also shows that the sample loading zones (30) of the assay test strips are oriented toward the base (1) of the fully assembled container. As the assay sample fluid in the container contacts the sample loading zone (30), it migrates upward through the assay test strip. Id. at 6:55–67. In another embodiment, multiple test strips may be held within a single "continuous in width" flow control channel.
 Exemplary Claim 3 at issue in this appeal reads as follows (wherein the language of independent claim 1 is distinguished by being in italics):
Exemplary Claim 3 at issue in this appeal reads as follows (wherein the language of independent claim 1 is distinguished by being in italics):
3. A device [according to claim 1] for collecting and assaying a sample of biological fluid, the device comprising:
(a) a flow control channel defined by at least one liquid pervious side joined to liquid impervious sides, wherein the internal dimensions of the flow control channel are sufficient to permit placement therein of an assay test strip;
(b) an assay test strip within the flow control channel, wherein the assay test strip has a sample loading zone therein, and wherein further the assay test strip is disposed within the flow control channel so the sample fluid contacts the sample loading zone at a liquid pervious side of the flow control channel; and,
(c) a sample fluid container having a base, an open mouth, and walls connecting the base to the mouth;
wherein the flow control channel is disposed inside the sample fluid container with the liquid pervious side oriented the base of the sample fluid container so that the assay sample fluid, when added to the container, is delivered to the sample loading zone of the assay test strip by entry through a liquid pervious side of the flow control channel without migration through an intermediate structure, and wherein entry of fluid into the flow control channel creates an ambient pressure within the flow control channel equivalent to the ambient pressure outside of the flow control channel, thereby eliminating a pressure gradient along which excess sample fluid could flow into the flow control channel.
wherein one of the liquid impervious sides of the flow control channel is formed as a portion of a liquid impervious backing; and wherein the device farther comprises a holder fittable inside the fluid sample container, the holder having at least one slot formed therein to receive the backing.
The prior art cited by the Petitioner comprised two combinations of prior art patents: the first, U.S. Patent No. 5,656,502 (the '502 patent) in view of U.S. Patent No. 5,985,675 (the '675 patent) or U.S. Patent No. 5,602,040 (the '040 patent) and the second, U.S. Patent No. 6,379,620 (the '620 patent) in view of the '502 patent or U.S. Patent No. 5,500,375 (the '375 patent). As explained in the opinion, the '502 patent discloses a device that holds only one test strip, wherein one end of the strip holder is open permitting liquid to enter the device and come into contact with the test strip. The '675 patent discloses the additional feature that such devices can contain more than one test strip that can be used for a number of different assays, and the '040 patent discloses that the device can "incorporate two or more discrete bodies of porous solid phase material, e.g., separate strips or sheets, each carrying mobile and immobilised reagents."
In the second combination, the '620 patent discloses test strips oriented upwards towards the container mouth (which is in the opposite direction to the claimed invention), and the '375 patent discloses one or a plurality of test strips that are contained in a sealed holder laminate that does not require extraneous wicks for augmenting sample migration, wherein multiple tests can be performed at the same time.
The procedural history of the case began when Rembrandt asserted the '019 patent against Alere in district court proceedings for infringement (this action remains stayed while the IPR proceedings are concluded). In response, Alere filed an IPR petition, wherein in that IPR the Board did not institute against all '019 patent claims and Rembrandt disclaimed claims 1, 9, and 11-15 of the '019 patent. As a consequence the Board instituted only as to claims 2-5. The Board found claim 2 to be invalid for anticipation by the '675 patent but that the challenger failed to establish invalidity for the other claims. In the appeal from that Board decision the Federal Circuit affirmed the Board's claim construction but remanded for consideration of all challenged claims under SAS Institute Inc. v. Iancu.
On remand, Rembrandt argued that Alere raised new theories in reply to patentee's patent owner statement, and that through its expert witness had proposed three new theories of invalidity. As noted in the opinion, these objections were not raised against obviousness rejections based on either of the three combinations of references cited by the panel here. The Board rejected Rembrandt's objections and found in its Final Written Decision that claims 2-6 and 10 were unpatentable, once again finding claim 2 to be anticipated by the '675 patent and the remaining claims to be obvious. The Board's reasoning for the obviousness of claims 3-6 credited Alere's expert testimony (Rembrandt did not rebut with an expert witness of its own) that, based on the second combination of references asserted by Alere the skilled worker would have modified the disclosure of the '620 patent "to remove the wicking material and re-orient the flow control channels towards the bottom of the container." This would have the advantage, Alere persuasively argued, of "reduc[ing] cost, complexity, and oversaturation of the test strip" based on the unrebutted testimony of its expert. And as for claim 10, the Board credited the first combination of references for teaching modification of the single test strip embodiment disclosed in the '675 patent in view of the other references because the skilled worker would have recognized the advantages of multiple strips, an argument again supported by unrebutted testimony from Alere's expert witness.
The Federal Circuit affirmed, in an opinion by Judge Reyna joined by Chief Judge Moore and Judge Dyk. On appeal, Rembrandt argued that the Board abused its discretion in considering Alere's purportedly new theories and erred in its obviousness determinations because they were not supported by substantial evidence. The Federal Circuit rejected Rembrandt's abuse of discretion argument because, first, it was forfeited when it objected on this basis on one obviousness ground (not at issue here) but did not assert this objection against the Board's obviousness determinations based on either of the two combinations of references at issue before the Court. The panel rejected what the opinion term Rembrandt's "generic" objection in its brief (merely arguing the existence of their objection without expressly objecting to either of these reference combinations as it had to a third). Part of the panel's decision was based on lack of notice and unfairness to both parties for the Board to read Rembrandt's objection this broadly. An additional ground for rejecting Rembrandt's abuse of discretion objection is the Court's recognition of the procedural and strategic context of IPR proceedings, where the Court has not permitted the Board "to consider a new theory of unpatentability raised by petitioner in reply or a new theory of patentability raised by patent owner in surreply," citing for example Intelligent Bio-Sys., Inc. v. Illumina Cambridge Ltd., 821 F.3d 1359, 1369 (Fed. Cir. 2016), and Henny Penny Corp. v. Frymaster LLC, 938 F.3d 1324, 1329–31 (Fed. Cir. 2019), while in other instances the Court held such consideration was within the proper scope of the Board's sound discretion, citing Ericsson Inc. v. Intell. Ventures I LLC, 901 F.3d 1374, 1379 (Fed. Cir. 2018); Chamberlain Grp., Inc. v. One World Techs., Inc., 944 F.3d 919, 925 (Fed. Cir. 2019); and Belden Inc. v. Berk-Tek LLC, 805 F.3d 1064, 1081–82 (Fed. Cir. 2015). In each of these cases whether the Board exercised its discretion properly or not was fact-specific and depended on whether considering such theories was fair to the parties. Important to the Court's determination of the Board's exercise of its discretion properly included, for example, whether a party "merely expand[ed] on a previously argued rationale as to why the prior art disclosures are insubstantially distinct from the challenged claims" (Ericsson) or whether a party was "elaborating on their arguments on issues previously raised" (Chamberland). More generally the panel acknowledged that these situation arose because "the very nature of the reply and sur-reply briefs are to respond (whether to refute, rebut, explain, discredit, and so on) to prior raised arguments within the confines of 37 C.F.R. § 42.23(b)" and thus can be proper. The Federal Circuit accordingly has held that “there is no blanket prohibition against the introduction of new evidence during an IPR," citing Anacor Pharms., Inc. v. Iancu, 889 F.3d 1372, 1380–82 (Fed. Cir. 2018), provided there is adequate notice to the other party, Genzyme Therapeutic Prods. Ltd. P'ship v. Biomarin Pharm. Inc., 825 F.3d 1360, 1366 (Fed. Cir. 2016).
Here, the Court found Rembrandt's arguments not to support an abuse of discretion by the Board, citing two examples. With regard to claim 10, the objection related to Alere's argument that modifying the '675 patent as suggested by the other cited references would be beneficial as to "cost and time savings." The panel recognized that in their petition Alere argued that modifying the device disclosed in the '675 patent would increase the "efficiency" of that device by permitting "multiple tests to be conducted simultaneously." Rembrandt countered in its Patent Owner's statement that the secondary references did not provide motivation for the combination, and Alere replied with the "cost and time savings" argument. The Federal Circuit considered this argument to have a "nexus" in Rembrandt's arguments in its Patent Owner's statement and was "a fair extension of [Alere's] previously raised efficiency argument" and thus was not an abuse of discretion for the Board to consider it. With regard to similar objections based on similar arguments Alere made in its Reply on the obviousness of claims 3-6, the Court held that Alere's arguments were responsive to Rembrandt's assertions in its Patent Owner statement; in addition in this case Alere was responding to the Board's original institution decision that the petition did "not explain sufficiently why modifying [the '620 patent] to remove the wicking material would have been understood to be beneficial." Finally, the Court rejected Rembrandt's objection on the grounds that Alere cited previously unidentified disclosure in certain of the cited references. The Court distinguished precedent where such arguments were prohibited (such as Apple and Ariosa Diagnostics v. Verinata Health, Inc., 805 F.3d 1359 (Fed. Cir. 2015)); the panel considered Alere's arguments to be "a legitimate reply" to Rembrandt's arguments because it was based on the same references and the same legal argument. Because ("in short") "Alere's responsive reply arguments do not constitute new theories," the Court held it was not an abuse of discretion for the Board to consider them.
The panel also rejected Rembrandt's other argument, that the Board's factual findings did not support its obviousness determinations. These arguments, the opinion states, "center on the interpretation of disclosures from the prior art and the presence of motivation to combine." What Rembrandt is lacking, the opinion notes, is any "counter testimony from a qualified declarant to refute Dr. Bohannon's conclusions regarding how [a skilled artisan] would have interpreted the identified disclosures." The Court notes that the Board was presented with "two alternative theories" and it was not the Court's "task 'to determine which theory [they] find more compelling,'" citing Shoes by Firebug LLC v. Stride Rite Childs. Grp., LLC, 962 F.3d 1362, 1371 (Fed. Cir. 2020). Thus, in the Court's opinion, the Board's obviousness determination were supported by substantial evidence.
Rembrandt Diagnostics LP v. Alere, Inc. (Fed. Cir. 2023)
Panel: Chief Judge Moore and Circuit Judges Dyk and Reyna
Opinion by Circuit Judge Reyna