
Perhaps the most significant Supreme Court decision in the past quarter century for the working patent practitioner is Dickinson v. Zurko, which strictly speaking is less a patent case than an administrative law decision. But every day the question of whether a party can successfully challenge a U.S. Patent and Trademark Office decision is decided on the basis of the Court's interpretation of the Administrative Procedure Act to require the Federal Circuit to give deference to the Office's factual determinations and only overturn them if there is not substantial evidence supporting them. In re Gartside, 203 F.3d 1305, 1316 (Fed. Cir. 2000). In a nonprecedential decision this week, Apotex v. Wyeth, the Federal Circuit once again applied the Court's instruction in upholding the Office's determination, in a Final Written Opinion from an inter partes review proceeding, that the claims at issue were not obvious.
The IPR involved U.S. Patent No. 7,879,828, which claims a stabilized formulation of tigecycline:
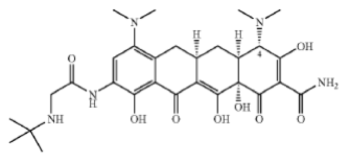
a tetracycline-class drug that is important as a treatment against drug resistant bacteria to be used when other antibiotics have failed. Tigecycline has a narrow stability profile, however, suffering oxidation at high pH and epimerization to an inactive form at acidic pH. The claimed formulation addresses these concerns by formulating the drug with an acid (in practice, hydrochloric acid) in the presence of a carbohydrate (lactose) that inhibits epimerization. Claim 1 is representative:
A composition comprising tigecycline, lactose, and an acid selected from hydrochloric acid and gentisic acid, wherein the molar ratio of tigecycline to lactose is between about 1:0.2 and about 1:5 and the pH of the composition in a solution is between about 3.0 and about 7.0.
The Board instituted IPR based on three references: a published Chinese patent application ("CN '550") that disclosed formulations of another tetracycline-type antibiotic, minocycline, as a powder of its hydrochloride salt in the presence of a "powder supporting agent" that could be a carbohydrate and in particular lactose. CN '550 was combined with a reference by Pawelczyk on the kinetics of minocycline oxidative degradation at pH greater than 5, and a reference by Naggar on the effects of stabilizers such as polysorbate 20, urea, and thiourea on tetracycline epimerization.
At trial, however, the Board found that Apotex had not established by a preponderance of the evidence that the claims of the '828 patent were obvious. The decision reasoned that Apotex had not shown why the skilled worker would have substituted tigecycline for minocycline in the composition disclosed in CN '550 nor why the skilled person would have prepared a lyophilized preparation of tigecycline according to the reference. The Board also found that Apotex had not shown why the skilled worker would combine the teachings of CN '550 with the Pawelczyk or Naggar references to arrive at the claimed formulation.
The Federal Circuit affirmed, in an opinion by Judge Lourie, joined by Judges Wallach and Hughes. The importance of the Zurko decision is evident in the portion of the opinion setting forth the standards of review the panel used in arriving at its opinion:
We review the Board's legal determinations de novo, In re Elsner, 381 F.3d 1125, 1127 (Fed. Cir. 2004), and the Board's factual findings underlying those determinations for substantial evidence, In re Gartside, 203 F.3d 1305, 1316 (Fed. Cir. 2000). Obviousness is a question of law based on underlying factual findings, In re Baxter Int'l, Inc. 678 F.3d 1357, 1361 (Fed. Cir. 2012), such as what a reference teaches, In re Beattie, 974 F.2d 1309, 1311 (Fed. Cir. 1992), and whether a skilled artisan would have had a reason to combine references, see In re Hyon, 679 F.3d 1363, 1365–66 (Fed. Cir. 2012).
Apotex argued that the Board had imported an "epimeric stabilization" limitation into the claim in finding non-obviousness, which challenge the panel rejected based on the absence of such a limitation in the claim. The Board had expressly stated that, because the claims did not recite this limitation "'obviousness of the claims [could] be demonstrated without a showing of epimeric stability in the prior art'" (emphasis in the Federal Circuit opinion). Accordingly, the Federal Circuit found no basis for Apotex's argument that the Board had improperly relied upon such a limitation in its non-obviousness finding. And the panel stated that, to the extent the Board considered epimeric stabilization at all, it was in the context of determining the motivation to combine references (a factual determination under In re Hyon).
Apotex's second argument was that the Board failed to consider any motivation to combine "beyond the problem the patentee was trying to solve," contrary to the Supreme Court's decision in KSR International Co. v. Teleflex Inc., 550 U.S. 398 (2007). Regarding this argument (again, a question of fact), the panel noted that the Board had considered the motivation to combine but had found Apotex's evidence thereof "wanting." In this regard, particularly as the question related to the motivation to substitute minocycline for tigecycline, the Board rejected the evidence proffered by Apotex's expert and credited Wyeth's expert that the oxidation and epimerization rates of minocycline and tigecycline were known to differ. According to the opinion:
Thus, there can be no question that the Board considered the structural similarities between tigecycline and minocycline as a potential motivating factor for a skilled artisan to substitute tigecycline for minocycline in the CN '550 composition. The Board simply found that the record did not support that finding, and we decline to disturb its decision on appeal.
The Court also expressly rejected Apotex's reliance on Senju Pharm. Co. v. Lupin Ltd., 780 F.3d 1337, 1346 (Fed. Cir. 2015), for the proposition that the skilled worker would be motivated to "make a simple substitution of generational drugs." The panel stated that its decision in Senju was dependent on "very factual findings" in that case, which "are notably absent here."
The Board also found, and the panel did not disturb these findings, that the skilled worker would not have been motivated to combine the cited references because none of the references disclosed tigecycline; the Naggar and Pawelczyk references did not disclose lactose as a stabilizing agents; the CN '550 reference did not teach the use of lactose for epimerization stabilization; and Apotex did not make a showing why the skilled worker would use lactose based on the teachings of the Naggar reference, which taught polysorbate 20 instead of lactose.
It should also be noted that this decision represents something of a unicorn, involving an IPR against a pharmaceutical (and brought by a generic company challenger) instituted over asserted prior art and resulting in a finding of non-obviousness based on that same art. The decision suggests that factual distinctions persuasive to the Board may be useful by patentees as kryptonite against invalidation in IPR proceedings, particularly due to the difficulty in persuading a not always patent supportive Federal Circuit to reverse the Board in view of the substantial evidence standard of review. While clearly not always available as a strategy, patentees in IPR would be remiss if they did not use expert testimony and other factual evidence to rebut prima facie obviousness assertions used as the basis for the Board's institution of an IPR against them.
Apotex Inc. v. Wyeth LLC (Fed. Cir. 2016)
Nonprecedential disposition
Panel: Circuit Judges Lourie, Wallach, and Hughes
Opinion by Circuit Judge Lourie