Key Points
- President Biden’s eagerly-awaited executive order (EO) on artificial intelligence (AI) tasks the Department of Health & Human Services (HHS) with promoting responsible AI innovation, development and use, including addressing the potential risks associated with AI-enabled technologies in health care and use of AI in drug development.
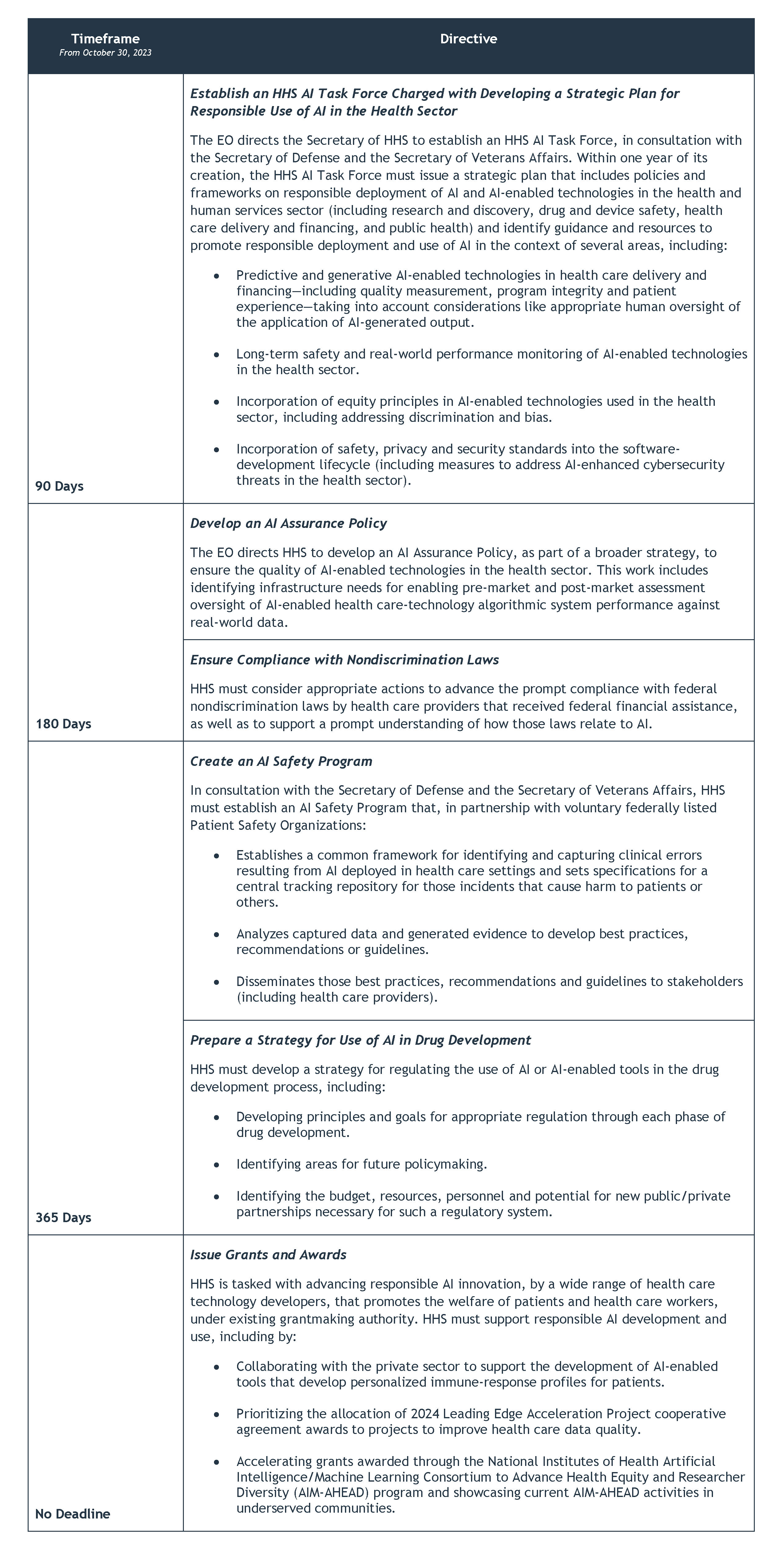
- The EO directs HHS to establish an AI Task Force, develop an AI Assurance Policy, consider actions to advance compliance with nondiscrimination laws, create an AI Safety Program, prepare a strategy for use of AI in drug development and issue grants and awards to encourage AI innovation and support responsible AI development and use.
- The EO should bolster the efforts the Food and Drug Administration (FDA) has already undertaken to regulate AI, and instructs HHS to take on the use of AI in technologies not under FDA’s purview.
Introduction
On October 30, 2023, the Biden Administration released an expansive EO on the Safe, Secure, and Trustworthy Development and Use of Artificial Intelligence1, which issues directives related to the use of AI across several sectors, including health care. Akin previously provided an overview of the EO here, and this article discusses the EO directives related to the health care sector.
The Biden Administration’s EO works to strike a balance between encouraging AI’s potential to transform technological innovation and protecting against the risks of unintended consequences. This perspective is consistent with the approach already taken by FDA, which has prioritized the development of policies specific to AI.2 In addition, the Office of the National Coordinator for Health Information Technology (ONC) proposed a sweeping Health IT Certification Program for “predictive decision support interventions,”3 which we wrote about here.
As with other sectors, the deployment of AI in the health care sector is well underway and development is outpacing federal efforts to regulate its use. For example, in 2021, AI/machine-learning (ML) was incorporated into one or more developmental stages for over 100 drugs and biologicals submitted to FDA,4 and as of October 19, 2023, FDA had reviewed 171 AI/ML-enabled medical devices.5 FDA has expressed the need for long-term safety and real-world performance monitoring of AI-enabled technologies; incorporation of equity principles in AI-enabled technologies; an incorporation of safety, privacy, security standards into the software-development lifecycle; and a tailored approach to reviewing the role of AI/ML in drug development—and the EO issues directives related to all of these considerations.
In addition to FDA, HHS will likely call upon several agencies and offices to carry out various aspects of the EO’s directives, including ONC, the Office for Civil Rights, the Centers for Medicare & Medicaid Services, the National Institutes of Health and the Office for Human Research Protections. In addition, the EO calls for extensive coordination among agencies in addition to HHS-led action items.
EO Directives for HHS
The EO calls on HHS to support the development of AI while safeguarding patients, consumers and workers by issuing the following directives:
Other EO Directives and Policies Impacting the Health Sector
The EO also includes instructions that apply across sectors that will especially affect health care, such as the directive that federal agencies use privacy-enhancing technologies (PETs) as appropriate. Directives aimed at managing AI in critical infrastructure and cybersecurity will also impact the health sector. In addition, some requirements apply directly to private companies, including those in the health care sector, such as the requirement that developers of “dual-use foundation models” submit reports to the Commerce Department outlining their training and testing procedures.
Moreover, several of the generally applicable guiding principles articulated in the EO are particularly relevant to health care. For example, the first guiding principle is that AI must be safe and secure, which resonates deeply in the health sector. The EO elaborates that in order to meet this goal, robust and standardized evaluations of AI systems will be needed to test, understand and mitigate risks of AI systems before they are put to use. The EO further emphasizes that testing and evaluations, including post-deployment performance monitoring, will be needed to help ensure that AI systems function as intended, are resilient against misuse or dangerous modifications, are ethically developed, are operated in a secure manner and are compliant with applicable laws.
The overarching guiding principles also cite privacy concerns and provide a reminder that otherwise applicable privacy requirements continue to apply in the AI context. The EO declares that the federal government will enforce existing laws and enact safeguards against infringements on privacy, noting that one critical field in which this is important is health care, where mistakes by, or misuse of, AI could harm patients. Indeed, privacy regimes implemented under HIPAA, HITECH and the FTC Act may continue to apply where data is created, received, maintained, transmitted or otherwise processed by AI systems.6
The EO also includes directives related to synthetic nucleic acids, including directing the Director of the Office of Science and Technology Policy, in consultation with the Secretary of HHS (among others), to establish a framework to encourage providers of synthetic nucleic acid sequences to implement comprehensive, scalable and verifiable synthetic nucleic acid procurement screening mechanisms, including standards and recommended incentives within 180 days. The EO also charges the Secretary of Commerce, within 180 days and in consultation with the Secretary of HHS (among others), to initiate an effort to engage with industry and relevant stakeholders to develop and refine for possible use by synthetic nucleic acid sequencers specifications for effective nucleic acid synthesis procurement screening and best practices for managing sequence-of-concern databases to support such screening. Furthermore, the EO also stipulates that within 180 days of the establishment of this framework, all agencies that fund life-sciences research shall establish that, as a requirement of funding, synthetic nucleic acid procurement is conducted through providers or manufacturers that adhere to the framework.
Next Steps
The EO provides opportunity for stakeholder engagement on AI issues related to health care and life sciences. As one example, the EO calls for collaborating with appropriate private sector actors through HHS programs that may support the advancement of AI-enabled tools that develop personalized immune-response profiles for patients.
The Akin Health Care & Life Sciences team regularly counsels clients that develop and deploy AI/ML technologies and will continue to monitor federal efforts to regulate the use of AI, including implementation of this EO.
1 The Administration also released a fact sheet highlighting key aspects of the EO, which is available at https://www.whitehouse.gov/briefing-room/statements-releases/2023/10/30/fact-sheet-president-biden-issues-executive-order-on-safe-secure-and-trustworthy-artificial-intelligence/.
2 FDA released an Action Plan for AI/Machine-Learning (ML)-Based Software as a Medical Device in January 2021, and two discussion papers on the use of AI and ML in drug development and manufacturing in May 2023. FDA, Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) Action Plan (Jan. 2021), https://www.fda.gov/media/145022/download; FDA, Using Artificial Intelligence and Machine Learning in the Development of Drug and Biological Products (May 2023), https://www.fda.gov/media/167973/download; FDA, Artificial Intelligence in Drug Manufacturing (May 2023), https://www.fda.gov/media/165743/download. FDA also recently announced a new Digital Health Advisory Committee to help the agency explore issues related to digital health technologies (DHTs), including AI/ML. FDA, Digital Health Advisory Committee, https://www.fda.gov/medical-devices/digital-health-center-excellence/fda-digital-health-advisory-committee (last updated Oct. 13, 2023).
3 Health Data, Technology, and Interoperability: Certification Program Updates, Algorithm Transparency, and Information Sharing, 88 Fed. Reg. 23746 (proposed Apr. 18, 2023) (to be codified at 45 C.F.R. pts. 170, 171).
4 FDA, FDA Releases Two Discussion Papers to Spur Conversation about Artificial Intelligence and Machine Learning in Drug Development and Manufacturing (May 10, 2023), https://www.fda.gov/news-events/fda-voices/fda-releases-two-discussion-papers-spur-conversation-about-artificial-intelligence-and-machine.
5 FDA, Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices, https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-aiml-enabled-medical-devices (last updated Oct. 19, 2023).
6 Specifically, these privacy regimes were adopted under the Health Insurance Portability and Accountability Act of 1996 (HIPAA), the Health Information Technology for Economic and Clinical Health Act of 2009 (HITECH), and Section 5 of the Federal Trade Commission Act (FTC Act).