Introduction: Harnessing Cellular Machinery for Precision Medicine
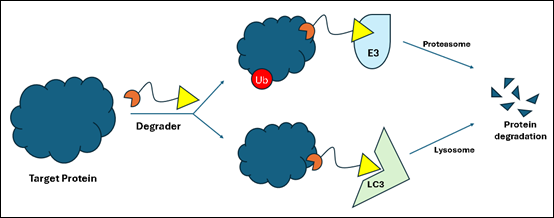
Targeted protein degradation (TPD) is transforming drug discovery by leveraging the cell’s natural protein disposal systems to eliminate disease-causing proteins. Innovators are making rapid and successful advancements in the field of targeted protein degradation, leaving intellectual property (IP) protections and strategies in hot pursuit. Unlike traditional inhibitors, which only modulate protein activity, protein degradation-based drugs induce the degradation of disease-related proteins, offering a fundamentally different approach to drug discovery. TPD has rapidly evolved from an academic concept to a key drug development strategy, with numerous candidates in preclinical and clinical pipelines. These therapies hold the potential to address previously “undruggable” targets – specifically, proteins lacking well-defined binding pockets – by harnessing endogenous degradation pathways.
There are multiple types of protein degraders, including (1) proteolysis-targeting chimeras (PROTACs); (2) molecular glues; (3) lysosome-targeting chimeras (LYTACs); (4) autophagy-targeting chimeras (AUTACs); and (5) autophagosome-tethering compounds (ATTECs). PROTACs and molecular glues are the most prevalent types, and have advanced the furthest in clinical trials.
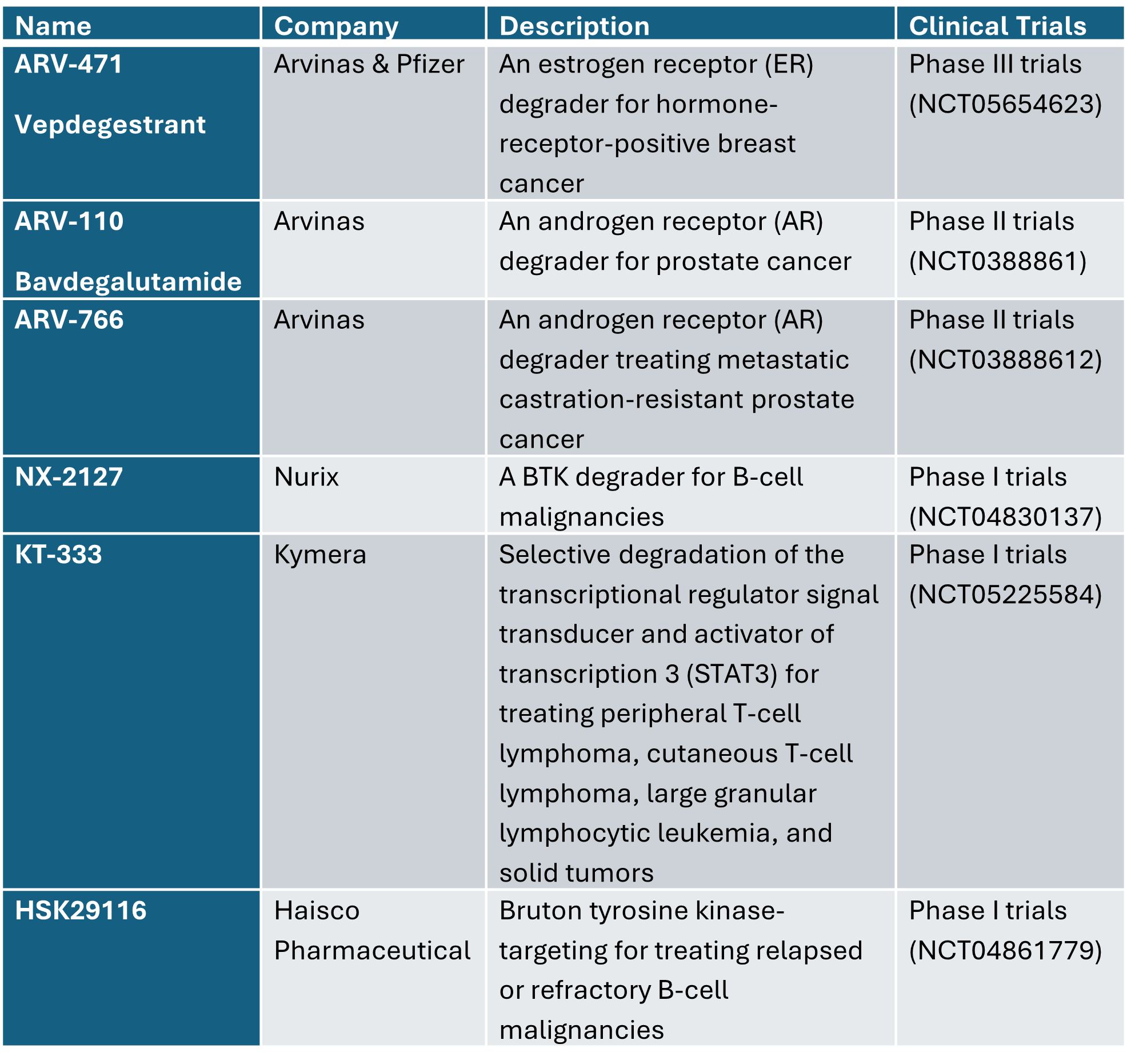
Examples of PROTACs in development:
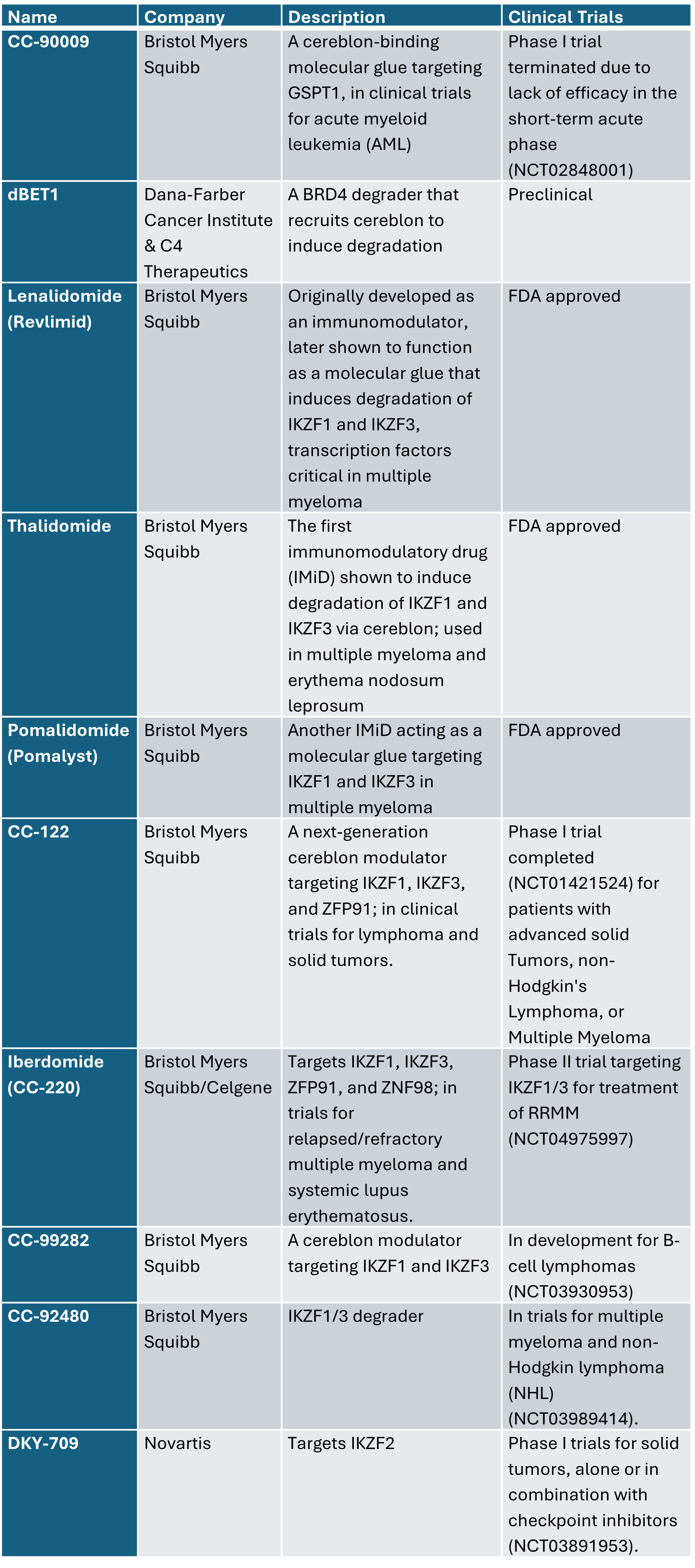
Examples of molecular glues in development:
Recent Developments:
- Arvinas’ ARV-471 showed a 30% ORR in interim Phase III data (Q1 2025), positioning it as a potential first-in-class PROTAC for breast cancer.
- Nurix’s NX-5948, a preclinical BTK degrader, entered IND-enabling studies for autoimmune indications, expanding TPD beyond oncology.
- Monte Rosa’s MRT-6160 initiated Phase I trials in Q1 2025, targeting VAV1 for autoimmune diseases, a novel application of molecular glues.
Despite its promise, targeted protein degradation faces significant scientific and practical challenges, including (1) E3 ligase selection and specificity;[17] (2) pharmacokinetics and stability;[18] (3) resistance mechanisms;[19] and (4) intellectual property and freedom to operate. These challenges have and will continue to inform the evolution of TPD.
As this modality matures, the IP and licensing environment around TPD is becoming increasingly complex. Foundational patents covering E3 ligase binders, linker chemistry, bifunctional molecule scaffolds, and specific degradation mechanisms mean that companies must navigate licensing agreements, potential litigation, and competitive patent filings in order to bring products to market. This dense and rapidly evolving patent thicket has implications for freedom to operate (“FTO”), strategic licensing, and patent litigation now, and for years to come. As a result, IP strategy is now a core competitive differentiator in the TPD space, influencing everything from early discovery to clinical development and corporate partnerships.
Key Players in the Targeted Protein Degradation Landscape
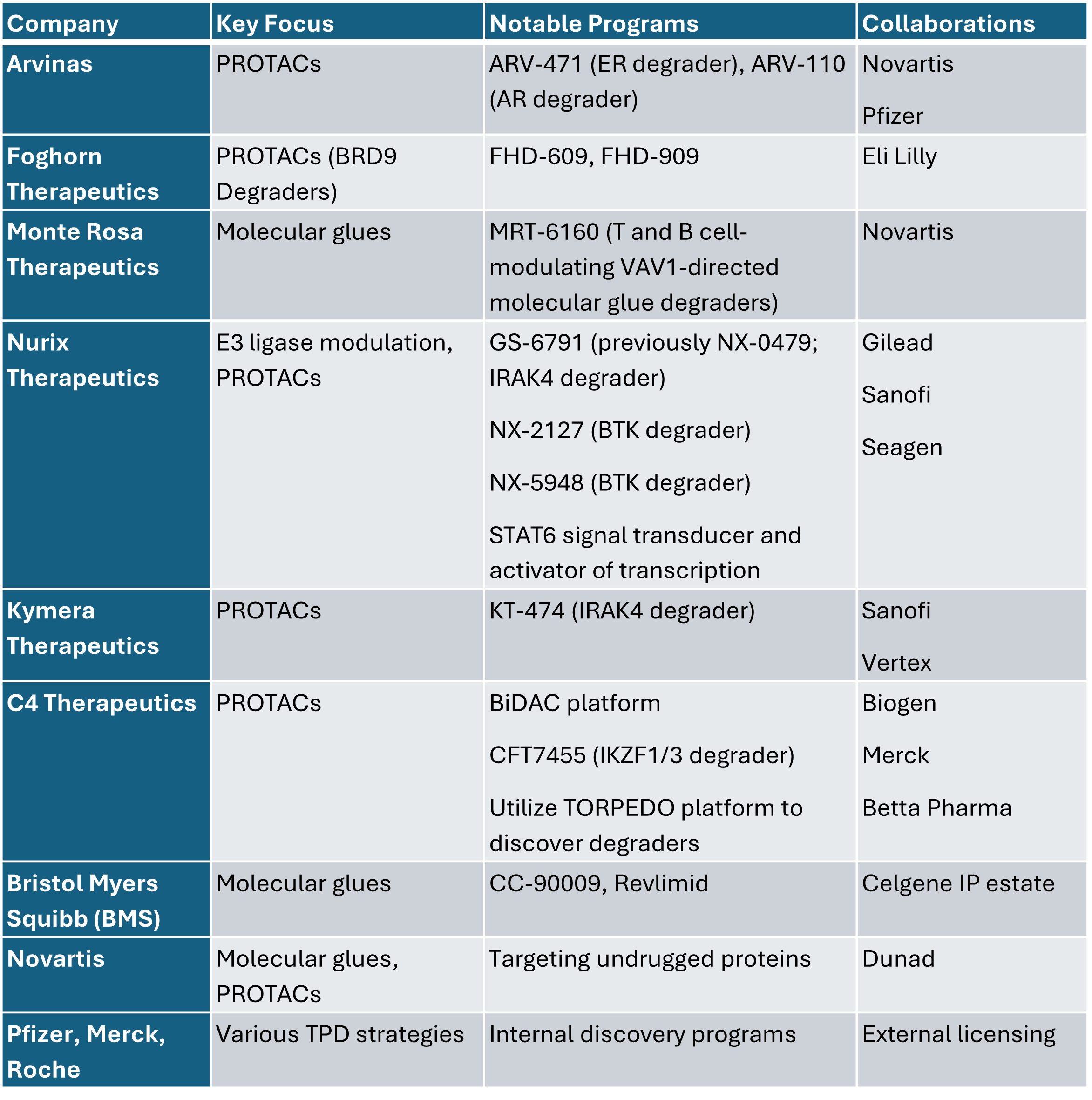
Several biotechnology and pharmaceutical companies are actively pursuing TPD therapies. The competitive landscape includes both dedicated TPD companies and large pharmaceutical firms incorporating degraders into their pipelines.
Companies with notable programs in the TPD field are provided in the table below.
Intellectual Property & Licensing Landscape in Targeted Protein Degradation
The field of targeted protein degradation (TPD) has witnessed significant growth, with numerous strategic partnerships and licensing agreements shaping its trajectory. These collaborations are pivotal in advancing TPD technologies and navigating the complex IP landscape.
- Intellectual Property Landscape
The IP landscape in TPD is becoming increasingly complex, with patents covering various aspects of the technology, including:
- Composition of Matter: Patents covering the specific chemical structures of PROTACs and molecular glues.
- Linker Chemistry: Innovations in linker design that connect the target-binding moiety to the E3 ligase recruiter.
- E3 Ligase Binders: Patents on ligands that recruit specific E3 ligases, such as cereblon or VHL.
- Degradation Mechanisms: Methods of inducing ubiquitination and subsequent proteasomal degradation.
As shown in the chart below, there has been a steady upward trend in the number of patents covering TPD, which shows that companies in this space have been increasing their patent filings.[34] From 2023 to 2024, the number of published TPD patents nearly doubled from 279 to 439 for U.S. and PCT applications.[35]
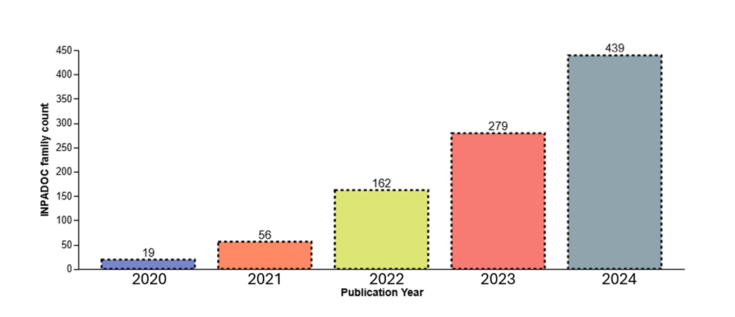
The assignees with the largest patent portfolios in the TPD space are shown below.[36]
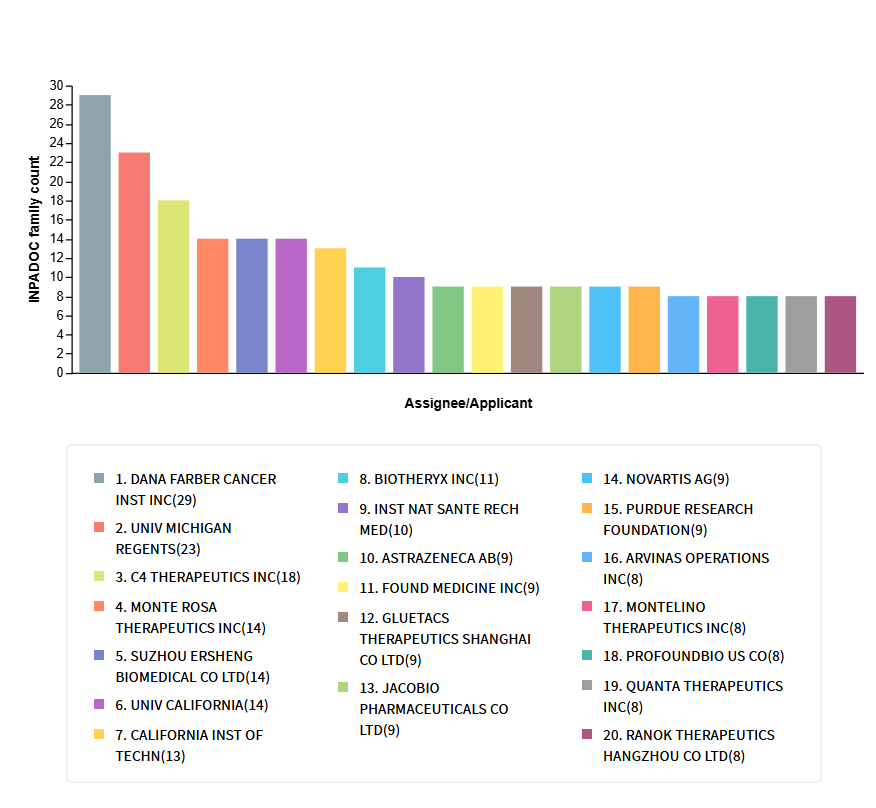
Players of all size have been involved in generating IP related to TPDs. For instance, Salarius Pharmaceuticals has expanded its IP portfolio with composition-of-matter protection into 2039 for novel molecular glues.[37] Kymera Therapeutics has been granted patents for compounds and methods aimed at the targeted degradation of proteins, addressing the treatment of disorders associated with target proteins.[38]
Indeed, a patent landscape analysis from January 2013 to July 2023 identified close to 622 INPADOC patent families relevant to PROTACs and bifunctional degraders from myriad assignees, highlighting the active IP management in this space.[39]
- Licensing
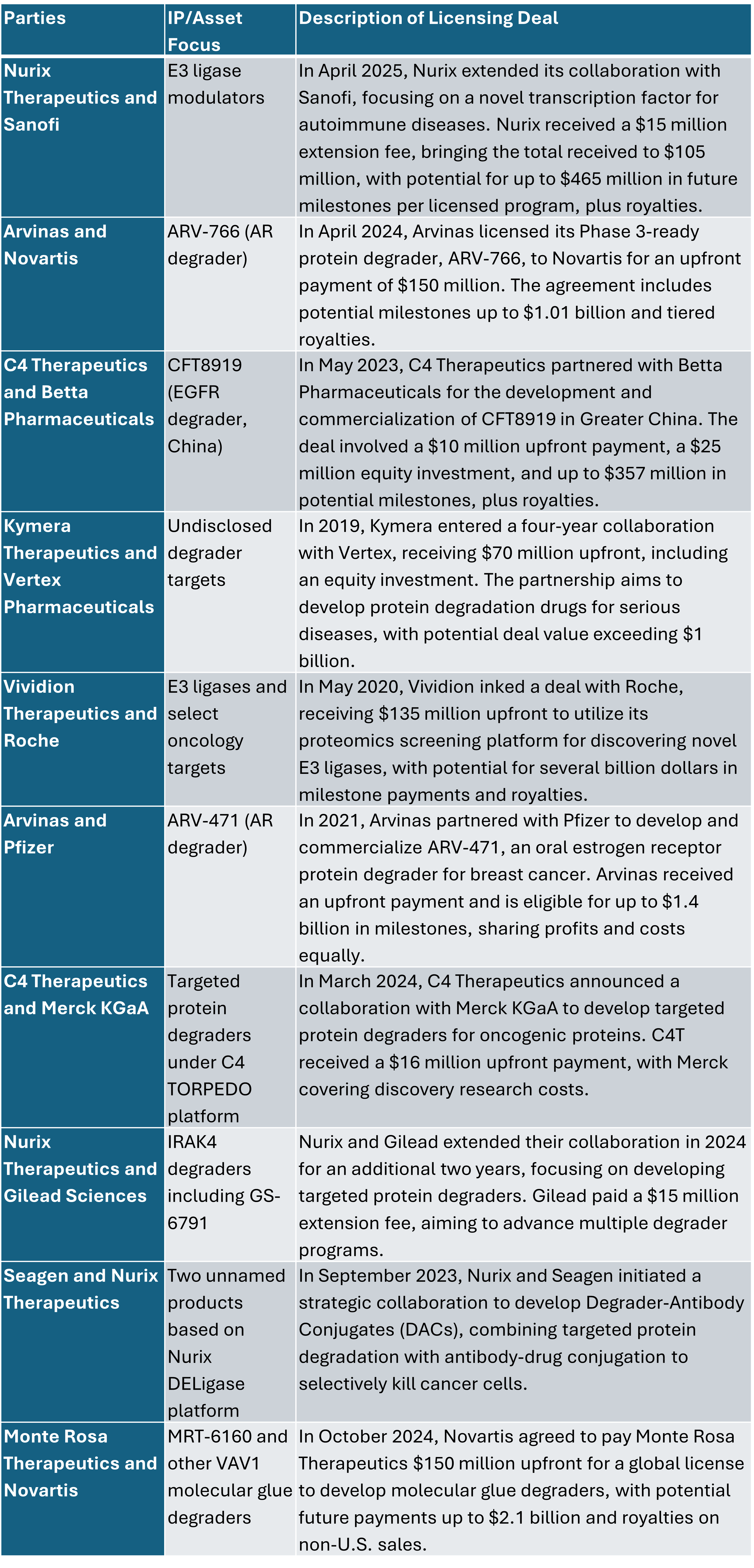
Because TPD is an innovative therapeutic approach that leverages the body’s natural protein disposal systems to eliminate disease-causing proteins with significant pharmaceutical possibilities, the field has garnered significant attention, leading to numerous strategic partnerships and licensing agreements aimed at advancing TPD technologies. Below is an overview of notable collaborations in this space.
Key Licensing Deals in Targeted Protein Degradation
These collaborations underscore the pharmaceutical industry’s commitment to advancing TPD technologies, aiming to address previously undruggable targets and develop novel therapies for various diseases. Further, large pharmaceutical companies are increasingly entering the space, leading to M&A and licensing deals.
Conclusion
Targeted protein degradation (TPD) is redefining the boundaries of drug discovery, offering transformative solutions for previously intractable diseases. With PROTACs, molecular glues, and emerging modalities like DUBTACs and LYTACs driving clinical successes, the TPD market is on track to explode higher. Yet, this promise comes with challenges: navigating a dense patent landscape, securing freedom to operate, and overcoming scientific and regulatory hurdles require strategic precision. As the TPD field accelerates, partnering with the patent counsel with subject matter expertise is critical to staying ahead.
[1] Tan, Xueqiang, et al. “Molecular glue-mediated targeted protein degradation: A novel strategy in small-molecule drug development.” Iscience (2024).
[2] Id.
[3] Id.
[4] Danilov, Alexey, et al. “A first-in-Human phase 1 trial of NX-2127, a first-in-class Bruton’s Tyrosine Kinase (BTK) dual-targeted protein degrader with immunomodulatory activity, in patients with relapsed/refractory B cell malignancies.” Blood 142 (2023): 4463.
[5] Tan et al.
[6] Tan et al.
[7] Hansen, Joshua D., et al. “CC-90009: a cereblon E3 ligase modulating drug that promotes selective degradation of GSPT1 for the treatment of acute myeloid leukemia.” Journal of medicinal chemistry 64.4 (2021): 1835-1843.
[8] Liu, Lei, et al. “Targeted BRD4 protein degradation by dBET1 ameliorates acute ischemic brain injury and improves functional outcomes associated with reduced neuroinflammation and oxidative stress and preservation of blood–brain barrier integrity.” Journal of neuroinflammation 19.1 (2022): 168.
[9] Yamanaka, Satoshi, et al. “Lenalidomide derivatives and proteolysis-targeting chimeras for controlling neosubstrate degradation.” Nature communications 14.1 (2023): 4683.
[10] Oleinikovas, Vladas, et al. “From thalidomide to rational molecular glue design for targeted protein degradation.” Annual review of pharmacology and toxicology 64.1 (2024): 291-312.
[11] Sasso, Janet M., et al. “Molecular glues: the adhesive connecting targeted protein degradation to the clinic.” Biochemistry 62.3 (2022): 601-623.
[12] Id.
[13] Id.
[14] Id.
[15] Hansen, Joshua D., et al. “Discovery of CRBN E3 ligase modulator CC-92480 for the treatment of relapsed and refractory multiple myeloma.” Journal of medicinal chemistry 63.13 (2020): 6648-6676.
[16] Id.
[17] Liu, Yuan, et al. “Expanding PROTACtable genome universe of E3 ligases.” Nature communications 14.1 (2023): 6509 (discussing how most PROTACs and molecular glues rely on a handful of E3 ligases, expanding to tissue-specific ligases could improve selectivity, but there may be need for better understanding of the >600 human E3 ligases).
[18] Poongavanam, Vasanthanathan, and Jan Kihlberg. “PROTAC cell permeability and oral bioavailability: a journey into uncharted territory.” Future Medicinal Chemistry 14.3 (2022): 123-126 (discussing that PROTACs are often large molecules (700-1200 Da) and the resulting challenges to traditional drug-like properties), which require solutions to issues with membrane permability, metabolic stability, and oral bioavailability).
[19] Burke, Matthew R., Alexis R. Smith, and Guangrong Zheng. “Overcoming cancer drug resistance utilizing PROTAC technology.” Frontiers in Cell and Developmental Biology 10 (2022): 872729 (discussing potential need for alternative ligases or degrader cocktails to mitigate resistance because tumor targets can develop resistance via mutations in target proteins, E3 ligases, or degradation pathways).
[20] https://ir.arvinas.com/news-releases/news-release-details/arvinas-enters-transaction-novartis-including-global-license
[21] https://www.pfizer.com/news/press-release/press-release-detail/arvinas-and-pfizer-announce-global-collaboration-develop
[22] https://foghorntx.com/wp-content/uploads/2023/04/Netherton_DDC_2023_presentation.pdf
[23] https://ir.monterosatx.com/news-releases/news-release-details/monte-rosa-therapeutics-announces-global-license-agreement
[24] https://ir.nurixtx.com/news-releases/news-release-details/nurix-therapeutics-outlines-2025-goals-and-objectives
[25] https://ir.nurixtx.com/news-releases/news-release-details/nurix-therapeutics-announces-extension-strategic-collaboration-0
[26] https://ir.nurixtx.com/news-releases/news-release-details/nurix-announces-strategic-collaboration-seagen-combining
[27] https://investors.kymeratx.com/news-releases/news-release-details/kymera-announces-expansion-kt-474-sar444656-hs-and-ad-phase-2
[28] https://investors.vrtx.com/news-releases/news-release-details/vertex-and-kymera-therapeutics-establish-strategic-collaboration
[29] https://ir.c4therapeutics.com/news-releases/news-release-details/c4-therapeutics-announces-delivery-second-development-candidate
[30] https://ir.c4therapeutics.com/news-releases/news-release-details/c4-therapeutics-announces-strategic-discovery-research
[31] https://ir.c4therapeutics.com/news-releases/news-release-details/c4-therapeutics-and-betta-pharmaceuticals-announce-exclusive
[32] https://news.bms.com/news/details/2019/Bristol-Myers-Squibb-to-Acquire-Celgene-to-Create-a-Premier-Innovative-Biopharma-Company/default.aspx
[33] https://www.biospace.com/novartis-strikes-1-3-billion-deal-with-protein-degradation-startup-dunad-therapeutics
[34] Search of Derwent database Apr. 2025 (INPADOC family count and publication year results generated using the keyword query: (CTB=((protein ADJ degrad*) OR (PROTAC OR proteolysis ADJ target* ADJ chimera) OR “molecular glue” OR (LYTAC OR lysosome ADJ target* ADJ chimera) OR (AUTAC OR autophagy ADJ target* ADJ chimera) OR (ATTEC OR autophagy ADJ teth* ADJ compound))) AND (AD>=(20200101)) AND (CC=(US OR WO)).
[35] Id.
[36] Search of Derwent database Apr. 2025 (INPADOC family count and assignee/applicant results generated using the keyword query: (CTB=((protein ADJ degrad*) OR (PROTAC OR proteolysis ADJ target* ADJ chimera) OR “molecular glue” OR (LYTAC OR lysosome ADJ target* ADJ chimera) OR (AUTAC OR autophagy ADJ target* ADJ chimera) OR (ATTEC OR autophagy ADJ teth* ADJ compound))) AND (AD>=(20200101)) AND (CC=(US OR WO)).
[37] https://www.biospace.com/salarius-pharmaceuticals-issued-u-s-patent-for-next-generation-targeted-protein-degraders
[38] https://www.pharmaceutical-technology.com/data-insights/kymera-therapeutics-gets-grant-for-targeted-degradation-of-proteins-for-treating-disorders/
[39] https://www.iam-media.com/article/leveraging-intellectual-property-bifunctional-protein-degraders-enter-clinical-trials
[40] https://ir.nurixtx.com/news-releases/news-release-details/nurix-licenses-drug-discovery-program-sanofi-targeting-novel
[41] https://ir.arvinas.com/news-releases/news-release-details/arvinas-enters-transaction-novartis-including-global-license
[42] https://ir.c4therapeutics.com/news-releases/news-release-details/c4-therapeutics-and-betta-pharmaceuticals-announce-exclusive
[43] https://investors.vrtx.com/news-releases/news-release-details/vertex-and-kymera-therapeutics-establish-strategic-collaboration
[44] https://vividion.com/news/vividion-therapeutics-announces-drug-discovery-collaboration-with-roche-focused-on-novel-e3-ligases/
[45] https://www.pfizer.com/news/press-release/press-release-detail/arvinas-and-pfizer-announce-global-collaboration-develop
[46] https://ir.c4therapeutics.com/news-releases/news-release-details/c4-therapeutics-announces-strategic-discovery-research
[47] https://ir.nurixtx.com/news-releases/news-release-details/nurix-therapeutics-announces-extension-strategic-collaboration
[48] https://ir.nurixtx.com/news-releases/news-release-details/nurix-announces-strategic-collaboration-seagen-combining
[49] https://ir.monterosatx.com/news-releases/news-release-details/monte-rosa-therapeutics-announces-closing-global-license