Two IPRs Filed Against Regeneron’s EYLEA® (aflibercept) Patent
On November 20, 2024, Samsung Bioepis filed IPR2025-00176 against Regeneron’s U.S. Patent No. 11,084,865 (“the ’865 patent”), challenging as obvious 48 claims (claims 1-12, 14-17, 19-20, 22-36, 39-42, 44-45, and 47-55) covering ophthalmic formulations of a vascular endothelial growth factor (VEGF) in vials and prefilled syringes. On December 2, 2024, Formycon filed IPR2025-00233 against the same patent, requesting joinder with Samsung Bioepis’s petition if it is instituted. In its conditional motion for joinder, Formycon requested that its petition be considered on the merits should Samsung Bioepis’s petition no longer be pending at the time its joinder motion is decided. These are the 23rd and 24th IPRs to be filed by biosimilars against EYLEA® (aflibercept) patents.
Samsung Bioepis’s Opuviz™ (aflibercept-yszy) and Formycon’s Ahzantive® (aflibercept-mrbb) are currently under preliminary injunctions preventing their commercial launch, based on the ’865 patent (previously reported Preliminary Injunctions Issued Preventing Launch of EYLEA® Biosimilars). Claims 4, 7, 9, 11, 14-17 of the ’865 patent were previously found valid by the District Court (Case No. 1:22-cv-00061 (N.D.W. Va.) / MDL 1:24-md-03103 (N.D.W. Va.)) in Regeneron’s litigation against Biocon and Mylan’s Yesafili™ (aflibercept-jbvf) (previously reported Permanent Injunction Issued Preventing Launch of EYLEA® Biosimilar Yesafili™). Appeals of the preliminary injunctions and validity opinion are ongoing.
Regeneron reported EYLEA® sales of $5.72 billion in 2023.
Herceptin® (trastuzumab) Biosimilar Hercessi™ Launch Announced
On November 29, 2024, Henlius announced that the first commercial shipment of Hercessi™ (trastuzumab-strf), a biosimilar of Genentech’s Herceptin® (trastuzumab), had been sent to the U.S. Herceptin® biosimilars have been available in the U.S. since July 2019, and Hercessi™ is the sixth to launch. According to Samsung Bioepis’s Q4 2024 Biosimilar Market Dynamics Report, Herceptin® biosimilars have achieved 86% market share during their time on the market.
Sixth Stelara® (ustekinumab) Biosimilar Approved: Biocon’s Yesintek™
On November 29, 2024, the FDA approved Biocon’s Yesintek™ (ustekinumab-kfce) as the sixth biosimilar of Janssen / Johnson & Johnson’s Stelara® (ustekinumab). Biocon previously settled with Janssen, agreeing to a launch date for Yesintek™ as early as February 22, 2025 (previously reported Stelara® Biosimilar Updates: Settlement of IPR and FDA Review of Proposed Biosimilar). The first Stelara® interchangeable biosimilar, Amgen’s Wezlana™ (ustekinumab-auub) is set to launch in the coming weeks, as early as January 1, 2025.
The table below shows the other approved and pending aBLAs for Stelara® biosimilars as well as their settlement market entry dates. There are no pending patent disputes related to Stelara® biosimilars.
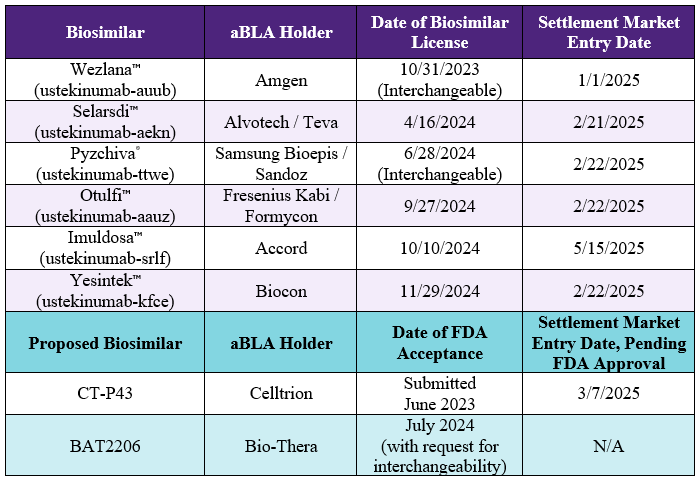
Johnson & Johnson reported Stelara® U.S. sales of $6.97B in 2023.
_____________________________________________________
The author would like to thank April Breyer Menon for her contributions to this article.