
Patent law is known for its several challenges in sufficiently capturing an invention, tangible in form and substance, in words with all their limitations. Patent law is known for being littered with traps for the unworthy. And patent law is replete with instances where claims are found unpatentable because they can fairly be construed to encompass disclosure in the prior art. All these features of patent law are present in one form or another in the Federal Circuit's decision handed down today in In re Qapsule Technologies, Inc.
The claims at issue in this appeal from the Patent Trial and Appeal Board (PTAB) decision affirming rejection are represented by rejected claim 1:
1. A synthetic capsule construct for providing a protected chemical milieu, the construct comprising:
a shell having a plurality of shell proteins, said plurality of shell proteins being assembled with one another for forming said shell and defining an enclosure therein, each of said shell proteins, when assembled for forming said shell, having an interior surface facing inwardly toward said enclosure and an exterior surface facing outwardly away from said enclosure, said shell serving to restrict permeability to and from said enclosure for providing the protected chemical milieu therein, said shell proteins being recombinant;
a cargo protein, said cargo protein being recombinant and optionally including a peptide tag; and
a bifunctional polynucleotide having both a first aptameric activity for binding said cargo protein and a second aptameric activity for retaining said bifunctional polynucleotide within said enclosure by assembly with the interior surface of said shell protein,
said bifunctional polynucleotide being non-naturally occurring; said bifunctional polynucleotide serving to link said cargo protein within said enclosure for providing the said cargo protein with the protected chemical milieu therein.
(Where the bold, italicized terms are identified in the Court's opinion as being relevant to the issues discussed therein.)
Procedurally before the Board Applicants overcame grounds of rejection under §§ 102 and 103 over references not discussed in the opinion, but (improvidently, as it turned out) when faced with new grounds of rejection from the PTAB decided not to reopen prosecution but to request rehearing under 37 C.F.R. § 41.50(B)(2). When that proved unavailing this appeal followed. The new grounds of rejection disclosed the following:
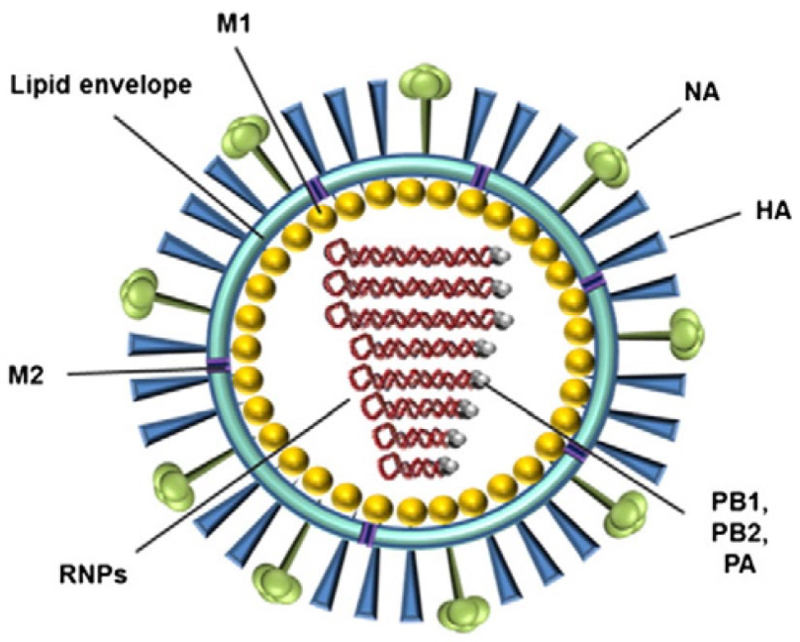 which the discerning will recognized to be an influenza virus (a recombinant one, as it turns out). The Board was able to map the limitations in the claims to this structure (wherein the M1 protein is a "shell protein," mutated viral protein PB1 satisfied the "cargo protein" limitation, and the interaction between the viral RNA and both the M1 protein and PB1 protein as part of the "mutated" (and thus "non-naturally occurring") viral RNPs constituted the "bifunctional polynucleotide" limitation. Finally, the Board rejected Applicant's argument that the reference did not disclose "recombinant" proteins as an attempt to impose a process limitation (how the proteins were made) on a product claim. Having found all the recited elements disclosed in this recombinant influenza virus, the Board affirmed its own determination that the Perez reference "inherently" anticipated the claimed invention.
which the discerning will recognized to be an influenza virus (a recombinant one, as it turns out). The Board was able to map the limitations in the claims to this structure (wherein the M1 protein is a "shell protein," mutated viral protein PB1 satisfied the "cargo protein" limitation, and the interaction between the viral RNA and both the M1 protein and PB1 protein as part of the "mutated" (and thus "non-naturally occurring") viral RNPs constituted the "bifunctional polynucleotide" limitation. Finally, the Board rejected Applicant's argument that the reference did not disclose "recombinant" proteins as an attempt to impose a process limitation (how the proteins were made) on a product claim. Having found all the recited elements disclosed in this recombinant influenza virus, the Board affirmed its own determination that the Perez reference "inherently" anticipated the claimed invention.
The Federal Circuit affirmed, in an opinion by Judge Newman joined by Judges Chen and Stoll. The panel recognized that Applicant had the burden to overcome the deference the Federal Circuit is compelled to give the PTO on fact questions (such as anticipation) and accordingly found that the Board had adduced sufficient evidence to support its rejection of these claims under § 102. Specifically, the Court rejected several distinctions drawn by Applicant because they were not affirmatively recited (despite being negative limitations). For example, to Applicant's argument that viral assembly as disclosed in the cited art requires viral ribonucleoproteins (RNPs), the Court notes that the claims recite that the claimed synthetic capsule construct "comprises" the recited elements (and in other limitations recites the open claim language "having") and thus does not exclude embodiments that include such RNPs. Accordingly, "it does not affect the Board's ground of rejection that Qapsule has purportedly created a capsule that will assemble in the presence of only a shell protein, cargo protein, and bifunctional polynucleotide, . . . for representative claim 1 is not so limited," and the panel held that the Board's conclusion was supported by substantial evidence. Similarly, the Court found unpersuasive other aspects of the recombinant influenza virus disclosed in the Perez reference not recited in the rejected claims (the viral lipid envelope, the other proteins comprising the virus) using the same reasoning, as well as arguments related to the different functions of the claimed construct and the prior art virus. These distinctions did not persuade the panel because "unclaimed functional distinctions or uses are insufficient to overcome anticipation," and, in the only faint glimmer of hope provided to Qapsule in the opinion, "[i]t is not before us to decide whether further specificity in the claims might distinguish these references."
It is not without some irony that the specification recites a great number of sources of shell and capsid proteins and viruses that produce them:
• [0008] Another preferred embodiment selects the shell protein from a group consisting of capsid proteins, coat proteins, and envelope proteins. In particular, the shell protein may be Qβ capsid protein; alternatively, the shell protein may be of a type derived from a single-stranded RNA virus, for example, icosahedral virus, bromovirus, comoviruses, nodavirus, picornavirus, tombusviruses, levivirus, or tymovirus. In another preferred embodiment, the shell protein is of a type derived from a double-stranded RNA virus, for example, birnavirus and reovirus. In another preferred embodiment, the shell protein is of a type derived from a double-stranded DNA virus, for example, parvovirus, microvirus, podovirus, or polyomavirus.
• [0083] However, capsid proteins from other bacteriophages and viruses may be employed assembling synthetic capsule constructs. The bifunctional polynucleotide employed with an alternative capsid protein employs an aptamer obtained from or adapted to assemble with such virus or bacteriophage shell proteins. Bifunctional polynucleotide employable with capsids from single-stranded RNA viruses and bacteriophages are single-stranded RNA. Exemplary single-stranded RNA viruses having assemblable shell proteins employable with the present invention are as follows
• [0084] Non-icosahedral viruses (rod-shaped or other shapes) tobacco mosaic virus.
• [0085] Bromoviruses: alfalfa mosaic virus, brome mosaic virus, cowpea chlorotic mottle virus, cucumber mosaic virus, tomato aspermy virus.
• [0086] Comoviruses: bean pod mottle virus, cowpea mosaic virus, tobacco ringspot virus.
• [0087] Nodaviruses: black beetle virus, pariacoto virus.
• [0088] Picornaviruses: coxsackievirus, echovirus, foot and mouth disease virus, rhinovirus 14, poliovirus.
• [0089] Tombusviruses: artichoke mottled crinkle virus, red clover necrotic mosaic virus, tomato bushy stunt virus.
• [0090] Leviviruses: bacteriophages MS2, FR, GA, PP7.
• [0091] Tymoviruses: physalis mottle virus, desmodium yellow mottle virus, turnip yellow mosaic virus.
• [0092] Shell proteins from virus particles that package double-stranded RNA can also be employed. However, their bifunctional polynucleotides will employ a single-stranded aptamer to bind/assemble with shell proteins. Exemplary double-stranded RNA viruses having assemblable shell proteins employable with the present invention are as follows:
• [0093] Birnaviruses: infectious pancreatic necrosis virus, infectious bursal disease virus.
• [0094] Reoviruses: reovirus, rice dwarf virus.
• [0095] Shell proteins from virus particles that package DNA can also be employed. These are constructed in the same way, by expression in cells using plasmids that drive the synthesis of both the shell protein and pieces of DNA (usually single-stranded) that associate with the protein and get packaged inside. Exemplary DNA viruses having assemblable shell proteins employable with the present invention are as follows:
• [0096] Parvoviruses: adeno-associated virus, canine parvovirus, feline panleukopenia virus, porcine parvovirus.
• [0097] Microviruses: bacteriophages phi-x 174, G4, alpha-3.
• [0098] Podoviruses: bacteriophages P22, T7, epsilon 15.
• [0099]Polyomavirus: SV40, Murine polyomavirus, Merkel cell virus.
And yet influenza virus (indeed, any paramyxovirus) is not disclosed. It is possible that Qapsule will pursue claims having a scope that can better differentiate claimed embodiments supported by its disclosure from prior art recombinant virus, if only for claims withdrawn pursuant to a restriction requirement. But the procedural history of this case and the PTAB's and Federal Circuit's decisions on the merits provide yet another cautionary tale of the difficulties and uncertainties occasioned by the patenting process.
In re Qapsule Technologies, Inc. (Fed. Cir. 2019)
Nonprecedential disposition
Panel: Circuit Judges Newman, Chen, and Stoll
Opinion by Circuit Judge Newman