The Federal Circuit exhibited the current status of its obviousness jurisprudence in affirming the District Court's determination that the asserted claims of U.S. Patent No. 8,410,131 were obvious in a decision handed down this week in Novartis Pharmaceuticals Corp. v. West-Ward Pharmaceuticals Int'l.
The case arose in ANDA litigation over Novartis's Afinitor product (everolimus, a rapamycin derivative; the structure of this compound is set out in claim 1 of the '131 patent) to treat renal cell carcinoma (RCC), a particularly resistant form of cancer. Novartis asserted claims 1-3 of the '131 patent, directed to methods of treating RCC with Afinitor:
1. A method for inhibiting growth of solid excretory system tumors in a subject, said method consisting of administering to said subject a therapeutically effective amount of a compound of formula I
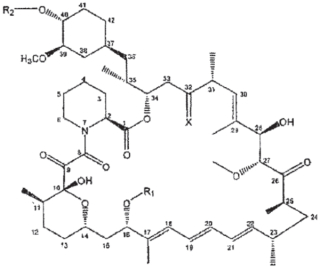
wherein
R1 is CH3,
R2 is —CH2—CH2—OH, and
X is =O.
2. The method of claim 1 wherein the solid excretory system tumor is an advanced solid excretory system tumor.
3. The method of claim 1 wherein the solid excretory system tumor is a kidney tumor.
As set forth in the Court's opinion, at the filing date of the '131 patent, "advanced RCC carried a poor prognosis and was known to be unpredictable and difficult to treat." Such treatment regimes that were available comprised various immunostimulants -- interleukin-2 and interferon-alpha among them -- but these compounds had "poor response rates and toxicity in patients." The history of attempts to develop a successful treatment strategy was, as the opinion characterized it, "unsuccessful." In this regard, the opinion also notes that most cancer drugs had a low success rate, "more than 70% of cancer drugs failing during phase II, and a majority of cancer drugs failing during phase III" clinical trials. (While a history like this should indicate that a defendant, burdened by the high "clear and convincing evidence" standard, should have a difficult time invalidating claims to a successful drug under obviousness, consider the Court's recent decision in Acorda Therapeutics, Inc. v. Roxane Laboratories, Inc.)
As the Federal Circuit explains in its opinion, everolimus is a species of a class of compound (including rapamycin, which has toxicities that preclude its use as an anticancer agent) termed mTOR inhibitors. In an explication of the mechanism of action of the drug (having dubious relevance to the bases of the Court's opinion), the Court explains that compounds like everolimus bind within a cell to FK506 binding protein (itself termed "FKBP-12") to form a complex, which binds to and inhibits the mTOR enzyme. At the filing date of the '131 patent, it was known in the art that mTOR inhibitors (like everolimus) were thought to inhibit hypoxia-inducible factor 1 (HIF-1) which were "hypothesized" to inhibit tumor growth, and another mTOR inhibitor, temsirolimus, had shown responses against RCC in phase I clinical trials. Rapamycin and its derivatives were known to have beneficial properties generally. As noted in the opinion, however, the art had no teaching that everolimus was effective as an antitumor agent ("let alone to treat advanced RCC").
With regard to RCC, the opinion notes that these tumors were "highly vascularized" and needed new blood vessels to be produced into the tumor (and without which the tumor cannot metastasize). This characteristic was relevant because vascularization is associated with expression of VEGF (vascular endothelial growth factor), which correlated with increased HIF-1 expression. While tantalizingly suggestive that inhibition of HIF-1 by mTOR inhibitors like everolimus might inhibit vascularization and thus have an antitumor effect against RCC, as the opinion notes at the '131 patent filing date:
HIF-1's precise mechanism of action and role in tumor growth were not yet fully understood. . . . Figure 4 [of a particular prior art reference to Semenza] disclosed that multiple genes (p53, PTEN, VHL), multiple pathways (RTKs, RAS, PI3K-AKT-FRAP, RAF-MEKERK), and multiple downstream effects (relating to VEGF, IGF-2, and glucose transporters) are associated with HIF-1 expression. While Semenza noted that "[i]t is possible that inhibition of HIF-1 activity may contribute significantly" to the anti-cancer effects of certain HIF-1 inhibitors, including rapamycin, it cautioned that the role of HIF-1 in RCC "requires further analysis."
Other prior art (to Zhong) showed that in vitro treatment of prostate cancer cells with mTOR inhibitors like rapamycin could inhibit HIF-1 expression (consistent with the general teaching in the art) and supported the hypothesis that "HIF-1α- dependent gene transcription . . . and the expression of a HIF-1-regulated gene product . . . are modulated by the activity of the PI3K/AKT/[mTOR] pathway in [prostate cancer] cells" and thus "may provide a basis for therapeutic efficacy," but "additional studies" were required.
In the context of these generic teachings in the art, West-Ward asserted four prior art references. Hidalgo 2000 disclosed development of rapamycin and temsirolimus (but not everolimus) as potential anticancer agents, tying mTOR inhibition by these drugs to interference with cell cycle progression pathways in tumor cells, leading to growth arrest (a goal of antitumor drugs). These suppositions were supported by results from two phase I clinical studies, which showed "major tumor responses" in RCC patients to temsirolimus, although the reference is appropriately cautious in extending its conclusions further than its results (which were limited to testing temsirolimus).
Hutchinson is a review of further studies with temsirolimus, including one study showing that, of 16 RCC patients treated with temsirolimus, one had a partial response and two others had a minor response to the drug; another study of 51 RCC patients showed 3 minor responses.
U.S. Patent No. 5,665,772 disclosed everolimus (in addition to other rapamycin derivatives) as being useful for many diseases and disorders ("organ transplant rejection, autoimmune diseases, asthma, and proliferative disorders such as tumors"). But "[i]t is undisputed that the '772 patent does not disclose any preclinical or clinical data on the antitumor activity of everolimus. It is also undisputed that the '772 patent does not contain an explicit disclosure that everolimus would be effective in treating advanced RCC."
Similarly, the final asserted reference, U.S. Patent No. 6,004,973, while disclosing "everolimus oral formulations, dosage ranges, and formulation techniques" did not disclose "any preclinical or clinical data showing any antitumor activity of everolimus, and does not disclose that everolimus would be effective in treating advanced RCC."
West-Ward's argument at trial was that what was known in the asserted art would have provided the skilled worker with a reasonable expectation of success in using everolimus to treat RCC (asserting the combination of either Hidalgo or Hutchinson with either the '772 or '973 patents). The District Court was unconvinced, finding that West-Ward had not established that the skilled worker would have been motivated to combine the references and specifically not finding any motivation to select, in particular, everolimus as an antitumor compound. The District Court recognized that everolimus would have been "one of several treatment options" for finding an effective treatment for aggressive solid tumors like RCC, but criticized West-Ward's expert for limiting his review of the prior art to mTOR inhibitors, amounting in the Court's opinion to hindsight bias. The District Court recognized "a variety of other treatments in development" at that time. The Court also credited "knowledge gaps" in the understanding of the molecular biology of advanced RCC that would have led the skilled worker to consider treatments other than mTOR inhibitors.
Turning to the other obviousness prong, a reasonable expectation of success, the District Court did not find that West-Ward had established that circumstance either. The asserted clinical data, limited to phase I trials, was insufficient to supply such an expectation according to West-Ward's own expert, and the lack of clinical data for everolimus, clinical differences between everolimus and temsirolimus, and the general failure rate of antitumor compounds taken together by the District Court led the Court to conclude that West-Ward had not shown that the cited art would have provided the skilled worker with the reasonable expectation of success required to establish obviousness by clear and convincing evidence.
The Federal Circuit affirmed in an opinion by Judge Stoll joined by Judges Plager and Clevenger. Although finding that the District Court had erred in finding there was no motivation to combine the asserted prior art, the Federal Circuit found no clear error regarding the District Court's finding that the skilled worker would not have had a reasonable expectation of success in using everolimus to treat RCC. Regarding the District Court's motivation to combine analysis, the panel considered the District Court's finding that a person of ordinary skill would have been "motivated to pursue everolimus as one of several potential treatment options for advanced solid tumors, including advanced RCC" sufficient for West-Ward to have established the requisite motivation to combine. The opinion further states that the District Court had improperly applied a heightened standard that required West-Ward "to prove that a person of ordinary skill would have selected everolimus over other prior art treatment methods." The panel rejected Novartis's assertion of Takeda Chemical Industries, Ltd. v. Alphapharm Pty., Ltd., 492 F.3d 1350 (Fed. Cir. 2007), as contrary precedent, on the ground that Takeda was a "lead compound" case and thus inapposite to this situation. "Lead compound" cases require a showing by clear and convincing evidence "that a person of ordinary skill 'would have had a reason to select a proposed lead compound or compounds over other compounds in the prior art,'" citing Daiichi Sankyo Co. v. Matrix Labs., Ltd., 619 F.3d 1346, 1354 (Fed. Cir. 2010) (emphasis added in opinion). The panel recognized other circumstances where the Court has determined whether there was a motivation to select a compound from a group, e.g., where the prior art discloses a range and the issue is whether there was a motivation to select a particular compound within the range; see Allergan, Inc. v. Sandoz Inc., 796 F.3d 1293, 1305 (Fed. Cir. 2015); Galderma Labs., L.P. v. Tolmar, Inc., 737 F.3d 731, 737–38 (Fed. Cir. 2013). None of these circumstances applied here, where Novartis asserted claims to methods of treating RCC with everolimus. Under these circumstances, the panel held that "[t]o the extent the district court required a showing that a person of ordinary skill would have selected everolimus over other prior art compounds, it erred." Indeed, the District Court's finding that the person of ordinary skill "would have been motivated to pursue everolimus as one of several potential treatment options for advanced solid tumors, including advanced RCC" was enough to satisfy the requirement that the cited art provided the needed motivation to combine.
Turning to the "reasonable expectation of success" prong, the Federal Circuit held that the District Court had correctly determined that the asserted art, in the context of the prior art, would not have given the skilled worker the reasonable expectation required. Specifically:
The district court correctly recognized that the temsirolimus phase I data resulted from small sample sizes and came from studies that were designed to test safety, not efficacy. It also noted that the studies disclosed in Hidalgo 2000 and Hutchinson do not reveal the total number of advanced RCC patients enrolled and that phase II data was not yet available. Further, it considered the testimony of West-Ward's expert Dr. Cho, who stated that a person of ordinary skill "would not make a determination or reasonable suggestion simply based in isolation upon whether a drug enters phase II," and who did not dispute that more than seventy percent of oncology drugs failed at phase II.
In addition to these deficiencies, the opinion notes the pharmacological differences between everolimus and temsirolimus, supported by expert testimony; that the prior art did not evince a full understanding of the relationship between mTOR inhibitors, HIF-1 expression, and tumor growth suppression, supported by unasserted prior art references before the District Court; and evidence that mTOR inhibition did not "necessarily result in tumor growth inhibition." On the totality of this evidence, the Federal Circuit found no evidence that the District Court erred in finding that West-Ward failed to establish that the art would have provided the skilled artisan with a reasonable expectation of success that everolimus could be used to treat RCC as claimed in the '131 patent, and thus affirmed the District Court's judgment.
Novartis Pharmaceuticals Corp. v. West-Ward Pharmaceuticals Int'l (Fed. Cir. 2019)
Panel: Circuit Judges Stoll, Plager, and Clevenger
Opinion by Circuit Judge Stoll