In earlier times, the Federal Circuit, responding to efforts by the U.S. Patent and Trademark Office to reject patent applications directed to biotechnology-related inventions, held (In re Brana) that utility of such inventions did not require demonstration of therapeutic effectiveness, those determinations being the purview of the FDA. Among other things, that apportionment of responsibilities was reaffirmed, albeit under different procedural circumstances, in the Federal Circuit's decision in United Therapeutics Corp. v. Liquidia Technologies, Inc.
The case arose in litigation between NDA holder United Therapeutics Corp. ("UTC") and Liquidia, who filed it own NDA (under § 505(b)(2) of the Food, Drug, and Cosmetic Act). Both regulatory approval applications were directed towards inhaled formulations of treprostinil for treating pulmonary hypertension (UTC's Tyvaso®, Liquidia's Yutrepia™). Relevant to the proceedings before the Federal Circuit, pulmonary hypertension (PH) presents in five subgroups, as explained in the opinion:
Group 1, pulmonary arterial hypertension ("PAH"); Group 2, pulmonary venous hypertension, i.e., pulmonary hypertension related to left-heart disease; Group 3, pulmonary hypertension associated with disorders damaging the lungs; Group 4, pulmonary hypertension caused by chronic thrombotic or embolic disease, including chronic blood clots in the lungs; and Group 5, a miscellaneous category for conditions that do not fit well into the other four subgroups.
A distinction between Group 2 and the remaining groups is that this malady arises due to cardiac issues ("postcapillary PH") while the rest of the groups are caused by pathologies in pulmonary capillaries ("precapillary PH"). Both parties' treprostinil formulations act by reducing pulmonary blood pressure by vasodilation.
UTC owns the Orange Book-listed patents at issue, U.S. Patent Nos. 9,593,066 and 10,716,793; representative claims of each patent are set forth in the opinion:
The '793 patent:
Claim 1. A method of treating pulmonary hypertension comprising administering by inhalation to a human suffering from pulmonary hypertension a therapeutically effective single event dose of a formulation comprising treprostinil or a pharmaceutically acceptable salt thereof with an inhalation device, wherein the therapeutically effective single event dose comprises from 15 micrograms to 90 micrograms of treprostinil or a pharmaceutically acceptable salt thereof delivered in 1 to 3 breaths.
The '066 patent:
Claim 1. A pharmaceutical composition comprising treprostinil or a pharmaceutically acceptable salt thereof, said composition prepared by a process comprising providing a starting batch of treprostinil having one or more impurities resulting from prior alkylation and hydrolysis steps, forming a salt of treprostinil by combining the starting batch and a base, isolating the treprostinil salt, and preparing a pharmaceutical composition comprising treprostinil or a pharmaceutically acceptable salt thereof from the isolated treprostinil salt, whereby a level of one or more impurities found in the starting batch of the treprostinil is lower in the pharmaceutical composition, and wherein said alkylation is alkylation of benzindene triol.
Claim 6. The pharmaceutical composition of claim 1, wherein the isolated salt is stored at ambient temperature.
Claim 8. A process of preparing a pharmaceutical product comprising treprostinil or a pharmaceutically acceptable salt thereof, comprising alkylating a triol intermediate of the formula:
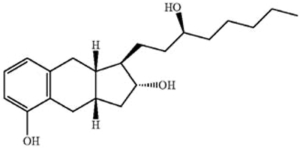 hydrolyzing the resulting compound to form treprostinil, forming a salt of treprostinil stable at ambient temperature, storing the treprostinil salt at ambient temperature, and preparing a pharmaceutical product from the treprostinil salt after storage, wherein the pharmaceutical product comprises Treprostinil or a pharmaceutically acceptable salt thereof.
hydrolyzing the resulting compound to form treprostinil, forming a salt of treprostinil stable at ambient temperature, storing the treprostinil salt at ambient temperature, and preparing a pharmaceutical product from the treprostinil salt after storage, wherein the pharmaceutical product comprises Treprostinil or a pharmaceutically acceptable salt thereof.
UTC brought suit asserting claims 1, 4, and 6-8 of the '793 patent and claims 1-3, 6, 8, and 9 of the '066 patent. UTC alleged that Liquidia's product, which has not been approved by the FDA and hence not marketed, would directly infringe the asserted claims of the '793 patent under 35 U.S.C. § 271(a) and induce infringement under 35 U.S.C. § 271(b). Liquidia counterclaimed that all asserted claims were invalid for failure to satisfy the written description and enablement requirements of 35 U.S.C. § 112(a). The District Court held that the asserted claims of the '793 patent were not invalid and that UTC has established by a preponderance of the evidence that Liquidia's product would infringe these claims directly and by inducement to infringe, rejecting Liquidia's argument that it lacked specific intent for the latter species of infringement. In this regard, the District Court found that administration instructions on Liquidia's label would "inevitably lead to the administration of a therapeutically effective single event dose" as recited in the asserted claims. Concerning Liquidia's counterclaims of invalidity, despite the art-recognized differences between PH Group 2 and the other groups of PH (and putative differences in safety and efficacy resulting therefrom), the District Court held that these considerations did not require undue experimentation by the skilled artisan because the claims did not require a showing of safety and efficacy. And the District Court found no failure to satisfy the written description requirement because the specification taught that "treprostinil would effectively vasodilate the pulmonary vasculature, improve hemodynamics, and treat a patient's elevated pulmonary blood pressure." Accordingly, the District Court pursuant to the statute stayed FDA approval until expiration of the '793 patent. (In a separate inter partes review proceeding brought by Liquidia, all claims of the '793 patent were found invalid, that decision being on appeal at the Federal Circuit. Should the Court affirm the PTAB's Final Written Decision of invalidity presumably this stay would be lifted.)
The District Court held that the asserted claims 1-3, 6, and 9 of the '066 patent were invalid due to anticipation by a prior art reference (Moriarty, which "discloses the synthesis of analogues of benzindene prostacyclins, including treprostinil"), and claims 1-3 would be infringed by Liquidia's treprostinil product but claims 6, 8, and 9 would not infringed (because Liquidia's product did not satisfy the "ambient temperature" limitation). Liquidia's counterclaims of invalidity for failure to satisfy the written description requirement, on the other hand, failed. This appeal followed.
The Federal Circuit affirmed the District Court's decision in all respects, in an opinion by Judge Lourie joined by Judges Dyk and Stoll; in so doing the opinion illustrates the difficulty appellants have in overcoming factual issues under the "clear error" standard in bench trials, the Federal Circuit repeatedly stating that the panel did not discern clear error in the District Court's factual findings. With regard to the '793 patent, the Federal Circuit rejected Liquidia's challenge to the District Court's claim construction of the term "treating pulmonary hypertension" not to require that such treatment be safe and efficacious based on the skilled artisan interpreting the claim to have these characteristics. This argument focused on treatment of Group 2 PH patients, wherein both parties' experts recognized that treprostinil would not benefit them (implicating efficacy at least for such treatments). While agreeing with the District Court that the phrase "treating pulmonary hypertension" included treating Group 2 PH patients (based on disclosure in the specification that did not distinguish between the PH groups in this regard), the District Court's construction of the phrase "a therapeutically effective single event dose of a formulation comprising treprostinil" (unchallenged by Liquidia), according to the Federal Circuit, did not incorporate into the claims "any additional efficacy limitations or any safety limitations." Without such a construction, the opinion asserts, "Liquidia's argument concerning the safety and efficacy of treating Group 2 PH patients is not before us" because "[q]uestions of safety and efficacy in patent law have long fallen under the purview of the FDA," citing In re Brana, 51 F.3d 1560, 1567 (Fed. Cir. 1995); Scott v. Finney, 34 F.3d 1058, 1063 (Fed. Cir. 1994); and In re Anthony, 414 F.2d 1383, 1395 (CCPA 1969). Accordingly, the Court refused to draw the distinctions Liquidia asked and thus the Federal Circuit affirmed the District Court's determination that Liquidia's treprostinil product would infringe the asserted claims of the '793 patent.
On similar bases, the Federal Circuit affirmed the District Court's determination that Liquidia did not establish by clear and convincing evidence than UTC's asserted claims were invalid for failing to satisfy either the written description or enablement requirements of 35 U.S.C. § 112(a). The panel held that the District Court had properly relied on expert testimony that "a skilled artisan would understand that the claimed administration of treprostinil would vasodilate the pulmonary vasculature, improve hemodynamics, and in this way for a single dose, treat a patient's elevated pulmonary blood pressure independent of the type (i.e., group) of pulmonary hypertension patient" and thereby satisfy the recited limitations in the asserted claims of the '793 patent. The Court sets forth these additional reasons for its decision regarding the § 112(a) requirements:
Liquidia essentially asks us to treat Group 2 PH as a claimed species within a larger genus (i.e., all five groups of pulmonary hypertension). But analogizing a subset of patients having a variant of a particular disease to traditional genus and species claims is inapt. It would be incorrect to fractionate a disease or condition that a method of treatment claim is directed to, and to require a separate disclosure in the specification for each individual variant of the condition (here, an individual group of pulmonary hypertension patients) in order to satisfy the enablement and written description provisions of 35 U.S.C. § 112, unless these variants are specified in the claims.
Further:
Disease-specific treatment requirements are matters for the FDA and medical practitioners. They are best suited to make these determinations because practitioners are informed by the findings of the regulatory agency to avoid treatment of patients who will not properly respond. And every claim to a method of treatment of an ailment has refinements. That is, for any given method of treatment claim, there may be a subset of patients who would not benefit from or should not take the claimed treatment. That does not mean that such claims are not sufficiently enabled or supported by written description. A subset of unresponsive patients is not analogous to unsupported species in a generic claim to chemical compounds.
On the question of inducement to infringe, the panel summarily rejected Liquidia's reliance on the PTAB's decision in a parallel IPR, that all claims of the '793 patent are invalid, because that decision is not yet final, distinguishing Commil USA, LLC v. Cisco Systems, Inc., 575 U.S. 632, 644 (2015). (This seems a sound application of judicial economy principles, because should the Federal Circuit affirm that PTAB determination Liquidia has a remedy in asking the district court to lift the stay on FDA approval of its commercial product.) On the merits, the panel agreed with UTC that all Liquidia's eventual label needs to provide is instructions to administer a therapeutically effective amount of treprostinil in a single event dose as required by the asserted '793 patent claims.
Turning to the '066 patent, the Federal Circuit deigned not to consider the parties' arguments regarding infringement based on the district court's determination that the asserted claims were invalid for anticipation by the Moriarty reference. UTC argued that the District Court erred in this determination, because the evidence was insufficient that Moriarty's pharmaceutical product contained the pattern of impurities in UTC's treprostinil formulation due to alkylation and hydrolysis steps in its preparation. The panel agreed with the District Court that the asserted claims were product-by-process claims that were evaluated for anticipation purposes as product claims, and the Moriarty reference showed the same level of impurities as found in UTC's treprostinil product. Finally, the Federal Circuit affirmed the District Court's finding that Liquidia's product did not infringe claims 6, 8, or 9 based on evidence that the product was stored at 2°-8°C and not ambient temperature as required by these claims.
For those keeping score, Liquidia is free of liability under the asserted claims of the '066 patent either because these claims are invalid (claims 1-3) or not infringed (claims 6,8, and 9). While Liquidia is precluded from obtaining FDA approval by the District Court's infringement determination of the asserted claims of the '793 patent, the existing stay on approval will likely be lifted if the Federal Circuit affirms the PTAB determination that these claim are invalid.
The Court' decision regarding safety and effectiveness appears somewhat paradoxical (at least with regard to efficacy) for claims reciting methods of treatment, particularly when further limited to a "single event dose." Some of the logic devolves to claim construction and Liquidia's failure to challenge the "therapeutically effective single event dose" limitation, while maintaining a bright line between the purviews of patent and regulatory law provide another reason. Further considerations involve the distinction the panel chose to draw between "unsupported species in a generic claim to chemical compounds" and "a subset of unresponsive patients" in a method of treatment claim. Whether these distinctions provide an avenue for applicants of the former class of claims to expand the scope of claims to generic chemical compounds is of course uncertain but perhaps provides a basis for the "clever draftsman" to work semantic magic (or legerdemain) to such ends.
United Therapeutics Corp. v. Liquidia Technologies, Inc. (Fed. Cir. 2023)
Panel: Circuit Judges Lourie, Dyk, and Stoll
Opinion by Circuit Judge Lourie